Nicotinamide Metabolic Dysregulation Plays a Key Role in Benzo[a]pyrene-Induced Adverse Birth Outcomes: Evidence from Multi-Omics Profiling, Experimental and Epidemiological Validation
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
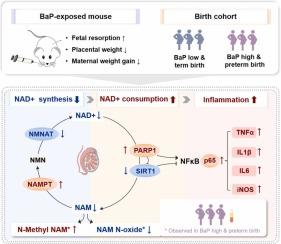
Prenatal exposure to Benzo[a]pyrene (BaP) has been shown to increase the risk of adverse birth outcomes, potentially through disruption of maternal metabolic homeostasis. However, the underlying pathways and mechanisms remain unclear. To address this knowledge gap, we employed a multi-omics approach combining experimental and epidemiological investigations. In the mouse model, untargeted metabolomics profiling revealed niacinamide (NAM) metabolism as the most significantly altered pathway in both maternal serum and placenta following BaP exposure. Mechanistically, BaP exposure reduced nicotinamide adenine dinucleotide (NAD+) synthesis while increasing its catabolism through activation of poly (ADP-ribose) polymerase 1 (PARP1) in placental tissue. Subsequent transcriptomic analysis showed that BaP-induced NAD+ depletion led to sirtuin 1 (SIRT1) suppression and consequent exacerbation of the NF-κB-mediated inflammatory response. These experimental findings were substantiated in human populations through targeted metabolomic analysis of a Chinese birth cohort, where significant NAM metabolic disturbances were observed in women with high BaP exposure experiencing preterm delivery. Our study provides novel mechanistic evidence that BaP-induced dysregulation of NAM metabolism mediates adverse birth outcomes through NAD+ deficiency and subsequent inflammatory response. These findings not only identify potential therapeutic targets but also highlight the urgent need for evidence-based environmental policy interventions and nutritional supplementation strategies to protect maternal-fetal health in high-risk populations.
烟酰胺代谢失调在苯并芘诱导的不良出生结局中起关键作用:来自多组学分析、实验和流行病学验证的证据
产前暴露于苯并[a]芘(BaP)已被证明会增加不良出生结果的风险,可能通过破坏母体代谢稳态来实现。然而,潜在的途径和机制尚不清楚。为了解决这一知识差距,我们采用了结合实验和流行病学调查的多组学方法。在小鼠模型中,非靶向代谢组学分析显示,在BaP暴露后,母体血清和胎盘中烟酰胺(NAM)代谢是最显著改变的途径。在机制上,BaP暴露减少了烟酰胺腺嘌呤二核苷酸(NAD+)的合成,同时通过激活胎盘组织中的聚(adp -核糖)聚合酶1 (PARP1)增加了其分解代谢。随后的转录组学分析显示,bap诱导的NAD+缺失导致sirtuin 1 (SIRT1)抑制,并随之加剧NF-κ b介导的炎症反应。通过对中国出生队列进行有针对性的代谢组学分析,这些实验发现在人群中得到了证实,在高BaP暴露的早产妇女中观察到显著的NAM代谢紊乱。我们的研究提供了新的机制证据,表明bap诱导的NAM代谢失调通过NAD+缺乏和随后的炎症反应介导不良的出生结果。这些发现不仅确定了潜在的治疗靶点,而且强调了迫切需要基于证据的环境政策干预和营养补充策略,以保护高危人群的母胎健康。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


