Hop bitter acids from the pistillate flower of Humulus lupulus L. and their anti-inflammatory and anti-dengue virus activities
IF 3.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Nine undescribed hop bitter acids, humulupulones A–I (1–9), along with three known ones (10–12) were isolated from the pistillate flower of Humulus lupulus L. (hops). Structurally, compounds 1 and 2 possess an unprecedented nitrogen-containing skeleton in α-acids, and this is the first time that nitrogen has replaced oxygen at the same position, thereby forming a new skeleton consisting of a fused α-acid and a dimethyl-substituted pyridine. Compound 3 is a rare pyridine-2,4-dione derivative simultaneously incorporating prenyl and isopropyl moieties. Compounds 7–10 were separated into four pairs of enantiomers by chiral HPLC separation. Their structures were unambiguously established by comprehensive spectroscopic analysis, X-ray crystallography, and ECD calculations. Moreover, compounds 2, 4, and 7 exhibited significant anti-inflammatory activities in the CuSO4-induced zebrafish inflammation model. Compounds 4, 5, 7–10 and 12 possessed significant anti-dengue virus (DENV) activities at the viral adsorption and entry stages with IC50 values ranging from 0.29 μM to 70.09 μM, while compounds 7, 10, and 11 exerted anti-DENV activities at the viral replication stage with IC50 values of 71.47 ± 7.30, 19.36 ± 6.77, and 16.18 ± 3.49 μM, respectively.
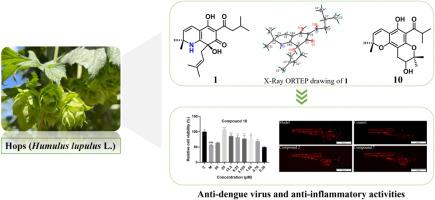
葎草雌蕊花啤酒花苦酸及其抗炎和抗登革病毒活性。
从葎草(Humulus lupulus L.,啤酒花)的雌蕊花中分离出九种未描述的啤酒花苦味酸,humulupulones A-I(1-9)和三种已知的啤酒花苦味酸(10-12)。在结构上,化合物1和2具有α-酸中前所未有的含氮骨架,这是氮首次在同一位置取代氧,从而形成由融合α-酸和二甲基取代吡啶组成的新骨架。化合物3是一种罕见的吡啶-2,4-二酮衍生物,同时含有戊烯基和异丙基。化合物7 ~ 10通过手性高效液相色谱分离成4对对映体。通过全面的光谱分析、x射线晶体学和ECD计算,明确了它们的结构。此外,化合物2、4和7在cuso4诱导的斑马鱼炎症模型中表现出显著的抗炎活性。化合物4、5、7-10和12在病毒吸附和进入阶段具有较强的DENV活性,IC50值为0.29 ~ 70.09 μM;化合物7、10和11在病毒复制阶段具有较强的DENV活性,IC50值分别为71.47±7.30、19.36±6.77和16.18±3.49 μM。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


