PI3K-inhibitory new anthraquinone-xanthone heterodimers from a deep-sea Epichloe sp. SCSIO 41042
IF 2.6
3区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
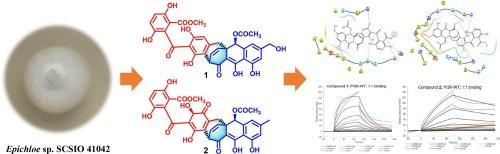
Two new xanthone-anthraquinone heterodimers (1 and 2), and eleven known anthraquinones (3−13) were isolated from the deep-sea derived Epichloe sp. SCSIO 41042 fermented on rice medium. Structurally, compounds 1–5 feature a unique bicyclo[3.2.2]nonane core formed through dual C![]() C linkages. Their structures and absolute configurations were determined by spectroscopic analyses and calculated electronic circular dichroism (ECD). Molecular docking was performed to characterize the affinity of anthraquinones for phosphatidylinositol 3-kinase (PI3K), identifying those with significant anti-PI3K activity. Active compounds were subsequently profiled via homogeneous time-resolved fluorescence (HTRF) assay for PI3K inhibition, followed by binding affinity validation using surface plasmon resonance (SPR). Activity assay revealed that 2 significantly inhibited both PI3KαWT and PI3KαH1047R enzymes, with IC50 values of 2.00 and 3.42 μM, respectively. Further SPR analysis demonstrated a moderate binding affinity between 2 and PI3K, with a KD of 12.7 μM.
C linkages. Their structures and absolute configurations were determined by spectroscopic analyses and calculated electronic circular dichroism (ECD). Molecular docking was performed to characterize the affinity of anthraquinones for phosphatidylinositol 3-kinase (PI3K), identifying those with significant anti-PI3K activity. Active compounds were subsequently profiled via homogeneous time-resolved fluorescence (HTRF) assay for PI3K inhibition, followed by binding affinity validation using surface plasmon resonance (SPR). Activity assay revealed that 2 significantly inhibited both PI3KαWT and PI3KαH1047R enzymes, with IC50 values of 2.00 and 3.42 μM, respectively. Further SPR analysis demonstrated a moderate binding affinity between 2 and PI3K, with a KD of 12.7 μM.
深海Epichloe sp.的pi3k抑制新蒽醌-山酮异二聚体SCSIO 41042。
从深海来源的Epichloe sp. SCSIO 41042中分离到2个新的黄酮-蒽醌异二聚体(1和2)和11个已知的蒽醌类化合物(3-13)。结构上,化合物1-5具有独特的双环[3.2.2]壬烷核,通过双CC键形成。通过光谱分析和计算电子圆二色性(ECD)确定了它们的结构和绝对构型。通过分子对接表征蒽醌类化合物对磷脂酰肌醇3-激酶(PI3K)的亲和力,鉴定出具有显著抗PI3K活性的蒽醌类化合物。活性化合物随后通过均匀时间分辨荧光(HTRF)分析PI3K抑制作用,然后使用表面等离子体共振(SPR)验证结合亲和力。活性测定结果显示,2对PI3KαWT和PI3KαH1047R酶均有显著抑制作用,IC50值分别为2.00和3.42 μM。进一步的SPR分析表明,2与PI3K之间的结合亲和力中等,KD为12.7 μM。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fitoterapia
医学-药学
CiteScore
5.80
自引率
2.90%
发文量
198
审稿时长
1.5 months
期刊介绍:
Fitoterapia is a Journal dedicated to medicinal plants and to bioactive natural products of plant origin. It publishes original contributions in seven major areas:
1. Characterization of active ingredients of medicinal plants
2. Development of standardization method for bioactive plant extracts and natural products
3. Identification of bioactivity in plant extracts
4. Identification of targets and mechanism of activity of plant extracts
5. Production and genomic characterization of medicinal plants biomass
6. Chemistry and biochemistry of bioactive natural products of plant origin
7. Critical reviews of the historical, clinical and legal status of medicinal plants, and accounts on topical issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


