Metabolite identification of ripretinib by UPLC-ESI-MS/MS: in silico prediction, in vitro metabolism, and stability assessment
IF 3.5
3区 医学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
Abstract
Ripretinib (RTB) is a switch-control tyrosine kinase inhibitor for advanced gastrointestinal stromal tumour (GIST) targeting receptor tyrosine kinase (KIT) or Platelet-Derived-Growth-Factor-Alpha (PDGFRα) mutations. Assessment of metabolic stability and characterization of drug metabolites are essential in determining the safety and risk assessment in clinical development. Initial site-of-metabolism predictions and biotransformation pathways were assessed using in silico tools. In vitro microsomal incubations in rat and human liver microsomes revealed Phase I metabolites, mainly by hydroxylation, and de-methylation using UPLC coupled with Orbitrap Exploris 120 HRMS/MS. Metabolic stability assessment showed that RTB has low intrinsic clearance and a relatively long half-life, indicating stable behavior. Furthermore, in silico toxicity predictions suggested an acceptable safety profile for RTB and its metabolites. Results highlighted neurotoxicity as a significant concern for RTB and its metabolites, while metabolite 1 exhibited hepatotoxicity and nephrotoxicity. The current study unveils metabolic profiles of RTB and helps to understand the biotransformation products-related toxicity.
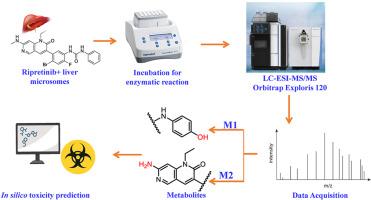
用UPLC-ESI-MS/MS鉴定利普雷替尼的代谢物:计算机预测、体外代谢和稳定性评估。
利普雷替尼(RTB)是一种开关控制酪氨酸激酶抑制剂,用于靶向受体酪氨酸激酶(KIT)或血小板衍生生长因子α (PDGFRα)突变的晚期胃肠道间质瘤(GIST)。在临床开发中,代谢稳定性评估和药物代谢物的表征对于确定安全性和风险评估至关重要。使用计算机工具评估初始代谢位点预测和生物转化途径。在大鼠和人肝微粒体的体外培养中,使用UPLC和Orbitrap Exploris 120 HRMS/MS,发现了主要通过羟基化和去甲基化产生的I期代谢物。代谢稳定性评估表明,RTB具有较低的内在清除率和较长的半衰期,表明其行为稳定。此外,硅毒性预测表明RTB及其代谢物具有可接受的安全性。结果显示,RTB及其代谢物主要表现为神经毒性,代谢物1表现为肝毒性和肾毒性。目前的研究揭示了RTB的代谢谱,并有助于了解生物转化产物相关的毒性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food and Chemical Toxicology
工程技术-毒理学
CiteScore
10.90
自引率
4.70%
发文量
651
审稿时长
31 days
期刊介绍:
Food and Chemical Toxicology (FCT), an internationally renowned journal, that publishes original research articles and reviews on toxic effects, in animals and humans, of natural or synthetic chemicals occurring in the human environment with particular emphasis on food, drugs, and chemicals, including agricultural and industrial safety, and consumer product safety. Areas such as safety evaluation of novel foods and ingredients, biotechnologically-derived products, and nanomaterials are included in the scope of the journal. FCT also encourages submission of papers on inter-relationships between nutrition and toxicology and on in vitro techniques, particularly those fostering the 3 Rs.
The principal aim of the journal is to publish high impact, scholarly work and to serve as a multidisciplinary forum for research in toxicology. Papers submitted will be judged on the basis of scientific originality and contribution to the field, quality and subject matter. Studies should address at least one of the following:
-Adverse physiological/biochemical, or pathological changes induced by specific defined substances
-New techniques for assessing potential toxicity, including molecular biology
-Mechanisms underlying toxic phenomena
-Toxicological examinations of specific chemicals or consumer products, both those showing adverse effects and those demonstrating safety, that meet current standards of scientific acceptability.
Authors must clearly and briefly identify what novel toxic effect (s) or toxic mechanism (s) of the chemical are being reported and what their significance is in the abstract. Furthermore, sufficient doses should be included in order to provide information on NOAEL/LOAEL values.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


