Ketalization of Glycerol with Acetone in the Presence of Natural Aluminosilicates Modified with Arenesulfonic Acid
IF 0.9
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
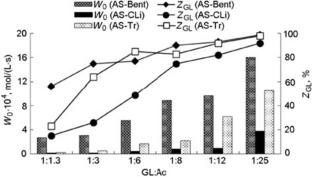
The kinetics of the reaction of glycerol with acetone in the presence of natural aluminosilicates modified with arenesulfonic acid fragments has been studied. The influence of the molar ratio of the reagents, reaction temperature, and the catalyst mass fraction on glycerol conversion and a rate of the accumulation of the main product (solketal) is established. The kinetic parameters of the reaction are determined.
丙烯磺酸改性天然硅酸铝存在下丙酮与甘油的酮化反应
研究了在天然硅铝酸盐经芳烃磺酸改性的情况下,甘油与丙酮的反应动力学。确定了反应物的摩尔比、反应温度和催化剂质量分数对甘油转化率和主要产物(solketal)积累速率的影响。测定了反应的动力学参数。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Theoretical and Experimental Chemistry
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
1.60
自引率
10.00%
发文量
30
审稿时长
6-12 weeks
期刊介绍:
Theoretical and Experimental Chemistry is a journal for the rapid publication of research communications and reviews on modern problems of physical chemistry such as:
a) physicochemical bases, principles, and methods for creation of novel processes, compounds, and materials;
b) physicochemical principles of chemical process control, influence of external physical forces on chemical reactions;
c) physical nanochemistry, nanostructures and nanomaterials, functional nanomaterials, size-dependent properties of materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


