Pyrrolizidine Alkaloids from Heliotropium lasiocarpum and Insecticidal Activity of Their Extracts
IF 0.9
4区 化学
Q4 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
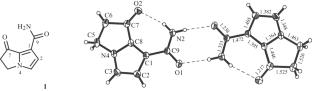
The new pyrrolizidine alkaloid helasine (1) and known heliotrine (2), lasiocarpine N-oxide (3), trachelanthamine (4), and echinatine (5) were isolated from the plant Heliotropium lasiocarpum. Alkaloids 4 and 5 were observed for the first time in H. lasiocarpum. The absolute configurations of isolated alkaloids 2–4 were established by X-ray crystal structure analyses. The insecticidal activity of the CHCl3, n-BuOH, and H2O parts of the H. lasiocarpum extract against Callosobruchus maculates and Sitophilus oryzae was examined. The CHCl3 extract at a dose of 10 mg/L had the highest activity.
龙葵中吡咯利西啶类生物碱及其提取物的杀虫活性
从植物Heliotropium lasiocarpum中分离得到新的吡咯利西啶类生物碱helasine(1)和已知的heliotrine(2)、lasiocarpine N-oxide(3)、trachelanthamine(4)和echinatine(5)。其中生物碱4和5为首次在石竹中发现。通过x射线晶体结构分析确定了分离得到的生物碱2 ~ 4的绝对构型。研究了水杨花提取物的CHCl3、n-BuOH和H2O部分对斑点胼手虫和米象虫的杀虫活性。CHCl3提取物在10 mg/L时活性最高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Natural Compounds
化学-有机化学
CiteScore
1.40
自引率
25.00%
发文量
265
审稿时长
7.8 months
期刊介绍:
Chemistry of Natural Compounds publishes reviews and general articles about the structure of different classes of natural compounds, the chemical characteristics of botanical families, genus, and species, to establish the comparative laws and connection between physiological activity and the structure of substances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


