Synthesis and Biological Evaluation of Isoaurone Derivatives as Anti-Inflammatory Agents
IF 0.9
4区 化学
Q4 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
A novel class of isoaurone analogs was designed, synthesized, and investigated in vivo for their anti-inammatory activity using a xylene-induced ear edema model. Isoaurones 8a–e exhibited anti-inflammatory activity at a dose of 100 mg/kg with inhibition rates ranging from 21.75% to 34.46%. 3-(4-(3-((5-(3-Chlorophenyl)-4H-1,2,4-triazol-3-yl)thio)propoxy)benzylidene)-6-methoxybenzofuran-2(3H)-one (8e) showed the most potent growth inhibitory effects (34.46%), indicated by the slightly higher inhibitory effects of celecoxib (31.92%).
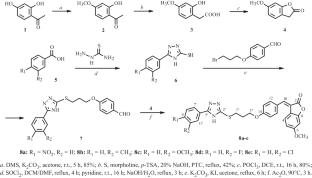
异aurone衍生物抗炎药物的合成及生物学评价
设计、合成了一类新的异aurone类似物,并利用二甲苯诱导的耳部水肿模型在体内研究了它们的抗炎活性。异aurones 8a-e在100 mg/kg剂量下表现出抗炎活性,抑制率为21.75% ~ 34.46%。3-(4-(3-(5-(3-氯苯基)- 4h -1,2,4-三唑-3-基)硫代)丙氧基)苄基)-6-甲氧基苯并呋喃-2(3H)- 1 (8e)的生长抑制作用最强(34.46%),塞来昔布的抑制作用略高(31.92%)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Natural Compounds
化学-有机化学
CiteScore
1.40
自引率
25.00%
发文量
265
审稿时长
7.8 months
期刊介绍:
Chemistry of Natural Compounds publishes reviews and general articles about the structure of different classes of natural compounds, the chemical characteristics of botanical families, genus, and species, to establish the comparative laws and connection between physiological activity and the structure of substances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


