Structure and properties of Cr–Ni–Ti–Fe coatings produced by non-vacuum electron beam surfacing technology
Abstract
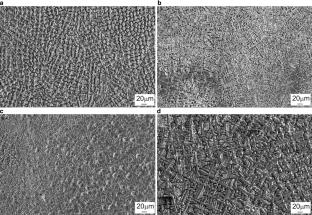
The structure and properties of coatings formed on the surface of structural steel are studied. Surfacing of Cr–Ni–Ti–Fe powder compositions is carried out using an ELV‑6 industrial electron accelerator providing the possibility of emitting electrons into an air atmosphere. This work sets out to develop high-quality surface layers with the properties corresponding to those of chromium-nickel austenitic steels. To that end, the phase composition and elemental distribution of alloying components in coatings are evaluated; structural features are analyzed; mechanical and adhesion characteristics of the deposited materials are determined. Coatings with a thickness of 1800–2700 µm are shown to be formed on the surface of steel. The coatings are characterized by a dendritic structure. According to X‑ray diffraction analysis, the dendrite grains are a γ-solid solution based on Ni and Fe. The maximum strength of ~900 MPa is observed in one-layer coatings. An increase in the concentration of alloying elements during the formation of two-layer coatings leads to a decrease in strength to 580 MPa. At the same time, the relative elongation is 28%. The adhesion strength of the coating with the base material reaches 560 MPa. In addition, alloying the surface of 12KhN3A steel with chromium and nickel makes it possible to increase its corrosion resistance in concentrated nitric acid.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


