Study of the electrochemical performance of highly Fe-doped SnO2 nanoparticles used as anode material for Li-ion batteries
Abstract
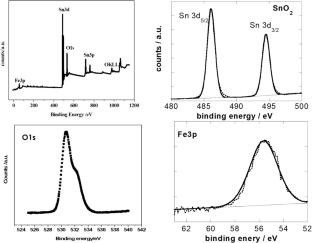
Iron (Fe)-doped SnO2 nanosized particles with various Fe amount were successfully elaborated to evaluate their performance as negative electrode for Li-ion battery. The XRD measurements reveal the rutile SnO2 structure for all doping concentrations together with the purity of the obtained phase. The NPs size for different Fe amounts as well as the lattice parameters and lattice volume are affected by Fe doping. HRTEM images further prove the nanoscale size of the obtained Fe-doped SnO2 particles. XPS measurements show the successful insertion of Fe as dopant in the SnO2 lattice without the formation of iron oxide phases and suggest that oxygen vacancies density is well affected by iron doping. Cyclic voltammetry results reveal an obvious dependence of the electrochemical activity on iron amount. These measurements suggest that Fe doping reduces the structural and textural changes undergone by SnO2 NPs during cycling. The galvanostatic charge/discharge curves shows that Fe doping improves the SnO2 cycling capability. This result is also demonstrated through the cycling performance test showing also that the optimal Fe concentration is around 10% for which the capacity reaches 555 mAh/g with a good cycling stability. Along with its effect on the specific area and the lattice parameters that affects the Li+ insertion/disinsertion rate, we suggested that the incorporation of Fe3+ ions stimulate the lithiation reactions which may increase the electrochemical property of SnO2 NPs. The measurements of the electrochemical impedance reveal that an optimal Fe amount for the electrochemical activity results from a competitiveness between the charge transfer resistance and the dielectric behavior of Fe-doped SnO2 NPs.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


