7,8-Dihydroxyflavone enhances the chemosensitivity of hepatocellular carcinoma cells to arsenic trioxide by disrupting mitochondrial oxidative phosphorylation
Pharmacological Research - Modern Chinese Medicine
Pub Date : 2025-09-20
DOI:10.1016/j.prmcm.2025.100693
引用次数: 0
Abstract
Introduction
Arsenic trioxide (ATO) has a well-documented history in traditional Chinese medicine as an effective treatment for acute promyelocytic leukemia and certain solid tumors; however, its efficacy as a standalone chemotherapy agent is limited. 7,8-Dihydroxyflavone (DHF), a TrkB receptor agonist, has shown promising anticancer effects across various malignancies. This study aimed to investigate the therapeutic potential of DHF, both alone and in combination with ATO, in hepatocellular carcinoma (HCC) cells.
Methods
The HCCLM3 and HepG2 cell lines were treated with ATO and DHF, alone or in combination. Cell viability and death ratio were assessed using MTT and live/dead staining. Proliferation and migration were evaluated through EdU staining, wound-healing assays, and colony formation assays. Apoptosis was detected via TUNEL staining, while changes in morphology and mitochondrial function were assessed using Mito-Tracker, transmission electron microscopy, mito-SOX, and JC-1 staining.
Results
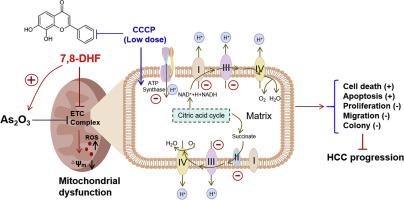
The combination of DHF and ATO significantly reduced cell viability, proliferation, and migration, while concurrently increasing cell death and apoptosis compared to monotherapy. Mechanistically, combination treatment notably decreased mitochondrial membrane potential, increased the production of mitochondrial reactive oxygen species (ROS), and suppressed mitochondrial respiration, which was accompanied by reduced protein levels of mitochondrial complexes I, II, III, and V. Furthermore, these effects were reversed by the mitochondrial uncoupler CCCP at a low dose.
Conclusion
These findings suggest that the DHF-ATO combination suppresses HCC progression by impairing mitochondrial oxidative phosphorylation, providing a promising strategy to enhance chemotherapeutic efficacy.
7,8-二羟黄酮通过破坏线粒体氧化磷酸化增强肝癌细胞对三氧化二砷的化学敏感性
三氧化二砷(ATO)作为一种治疗急性早幼粒细胞白血病和某些实体瘤的有效药物,在中医中有很好的记载;然而,作为单独的化疗药物,其疗效有限。7,8-二羟黄酮(DHF)是一种TrkB受体激动剂,在多种恶性肿瘤中显示出良好的抗癌作用。本研究旨在探讨DHF单独或与ATO联合治疗肝细胞癌(HCC)的治疗潜力。方法ATO和DHF分别单独或联合作用于HCCLM3和HepG2细胞株。采用MTT法和活/死染色法评估细胞活力和死亡率。通过EdU染色、伤口愈合试验和菌落形成试验来评估增殖和迁移。TUNEL染色检测细胞凋亡,Mito-Tracker、透射电镜、mito-SOX和JC-1染色检测细胞形态学和线粒体功能的变化。结果与单药治疗相比,DHF和ATO联合治疗显著降低了细胞活力、增殖和迁移,同时增加了细胞死亡和凋亡。从机制上看,联合治疗显著降低了线粒体膜电位,增加了线粒体活性氧(ROS)的产生,抑制了线粒体呼吸,同时线粒体复合体I、II、III和v的蛋白水平降低。此外,低剂量的线粒体解偶联剂CCCP可以逆转这些影响。结论DHF-ATO联合抑制HCC进展是通过损害线粒体氧化磷酸化,为提高化疗疗效提供了一种有希望的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


