Tailoring interfacial stability with lithium salt additives for long-cycling Ni-rich cathodes in lithium metal batteries
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
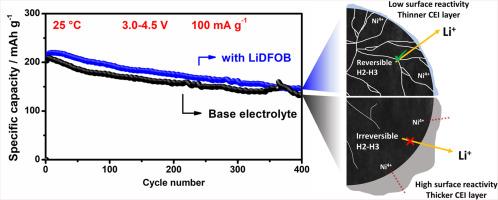
The development of next-generation battery systems with high energy density is crucial for the advancement of electric vehicles (EVs). The combination of a high-nickel cathode with a lithium-metal anode is a particularly promising candidate for this application, as both materials demonstrate individually high theoretical specific capacities. However, each electrode presents intrinsic challenges that must be individually understood and synergistically mitigated to unlock their full potential. In this study, the effectiveness of various lithium salts as electrolyte additives for stabilizing LiNi0.8Co0.1Mn0.1O2 (NCM811) cathodes is investigated. A systematic comparison of lithium tetrafluoroborate, lithium bis(oxalate)borate, lithium difluoro(oxalate)borate (LiDFOB), lithium nonafluoro-1-butanesulfonate, lithium bis(trifluoromethanesulfonyl)imide, and lithium trimethylsilanolate (LiTMS) is conducted in a LiPF6-based electrolyte to evaluate their impact on interfacial chemistry and long-term cycling performance. Among the additives tested, LiDFOB demonstrates the most promising results, retaining approximately 70 % of its initial capacity after 400 cycles at 25 °C and 87.7 % at 50 °C. In contrast, while LiTMS initially appears effective in forming a protective cathode electrolyte interphase (CEI), it ultimately leads to significant impedance growth and capacity degradation over extended cycling. Comprehensive electrochemical evaluations and ex situ characterizations reveal that the interfacial reactivity and integrity of the resulting CEI play a more critical role in determining long-term stability than the mechanical degradation traditionally attributed to particle cracking. These findings challenge the conventional emphasis on structural failure as the primary mode of degradation in high-Ni cathodes and underscore the significance of rational CEI design through electrolyte additive engineering.
锂盐添加剂用于锂金属电池中长循环富镍阴极的界面稳定性
下一代高能量密度电池系统的开发对电动汽车的发展至关重要。高镍阴极和锂金属阳极的组合是这一应用的一个特别有前途的候选者,因为这两种材料都具有很高的理论比容量。然而,每个电极都提出了内在的挑战,必须单独理解和协同缓解,以释放其全部潜力。本研究考察了不同锂盐作为电解质添加剂对LiNi0.8Co0.1Mn0.1O2 (NCM811)阴极的稳定性。在lipf6基电解质中,系统比较了四氟硼酸锂、双(草酸)硼酸锂、二氟(草酸)硼酸锂(LiDFOB)、非氟-1-丁烷磺酸锂、双(三氟甲磺酰)亚胺锂和三甲基硅酸锂(LiTMS),以评估它们对界面化学和长期循环性能的影响。在测试的添加剂中,LiDFOB表现出最有希望的结果,在25°C和50°C下循环400次后,其初始容量保持在70%左右,在50°C下保持在87.7%。相比之下,虽然LiTMS最初在形成保护性阴极电解质界面(CEI)方面表现有效,但它最终会导致显着的阻抗增长和容量下降。综合电化学评价和非原位表征表明,与传统上由颗粒破裂引起的机械降解相比,CEI的界面反应性和完整性在决定长期稳定性方面起着更关键的作用。这些发现挑战了传统的强调结构破坏是高镍阴极降解的主要模式,并强调了通过电解质添加剂工程合理设计CEI的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Surfaces and Interfaces
Chemistry-General Chemistry
CiteScore
8.50
自引率
6.50%
发文量
753
审稿时长
35 days
期刊介绍:
The aim of the journal is to provide a respectful outlet for ''sound science'' papers in all research areas on surfaces and interfaces. We define sound science papers as papers that describe new and well-executed research, but that do not necessarily provide brand new insights or are merely a description of research results.
Surfaces and Interfaces publishes research papers in all fields of surface science which may not always find the right home on first submission to our Elsevier sister journals (Applied Surface, Surface and Coatings Technology, Thin Solid Films)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


