Multitarget insights into traditional Chinese phytoconstituents against tuberculosis via network pharmacology, molecular docking, and MD simulation
Pharmacological Research - Modern Chinese Medicine
Pub Date : 2025-09-20
DOI:10.1016/j.prmcm.2025.100695
引用次数: 0
Abstract
Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis, is a global health threat. Traditional Chinese medicine (TCM), with its extensive use of plant-based compounds, offers a promising alternative for TB treatment. However, the molecular mechanisms underlying the effects of TCM phytoconstituents on TB have not been fully elucidated. This study aims to explore the multitarget therapeutic potential of TCM phytoconstituents for TB management using network pharmacology, molecular docking, and molecular dynamics (MD) simulations.
Methods
Network pharmacology was employed to identify the interactions between phytoconstituents and proteins associated with TB. Molecular docking evaluated compound binding to key TB targets, while MD simulations assessed complex stability and dynamics. Gene expression was analyzed using the eFP server, and mycoCSM predicted bacterial protein responses to compounds, indicating potential drug resistance or susceptibility.
Results
Network pharmacology analysis identified key biological pathways, including the prolactin signalling pathway and the phosphatidylinositol 3-kinase signalling, that may be modulated by these compounds. The docking scores range from -6.5 to -9.0 kcal/mol for Glabroisoflavanone A and B against three major proteins, viz. 1O43, 5XGI, and 6NJS. Both phytoconstituents exhibited a good anti-tubercular sensitivity score. MD simulations (200 ns) further revealed that Glabroisoflavanone A formed the most stable complex with 5XGI.
Discussion
The Glabroisoflavanone A-5XGI complex showed the strongest binding, supported by the most binding free energy (–75.19 ± 4.89 kcal/mol), suggesting a robust interaction. These findings highlight the differential binding and dynamic behavior of phytoconstituents, offering potential insights for therapeutic development.
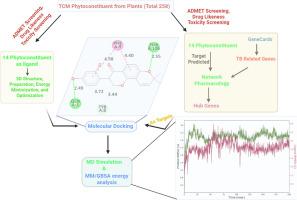
通过网络药理学、分子对接和MD模拟研究中药抗结核多靶点
由结核分枝杆菌引起的结核病是一种全球性的健康威胁。传统中药(TCM)广泛使用植物基化合物,为结核病治疗提供了一个有希望的替代方案。然而,中药植物成分治疗结核的分子机制尚未完全阐明。本研究旨在通过网络药理学、分子对接和分子动力学(MD)模拟,探索中药植物成分在结核病治疗中的多靶点治疗潜力。方法采用网络药理学方法鉴定结核相关蛋白与植物成分的相互作用。分子对接评估了化合物与关键结核靶点的结合,而MD模拟评估了复合物的稳定性和动力学。使用eFP服务器分析基因表达,mycoCSM预测细菌对化合物的蛋白质反应,表明潜在的耐药性或易感性。结果网络药理学分析确定了这些化合物可能调节的关键生物学通路,包括催乳素信号通路和磷脂酰肌醇3-激酶信号通路。Glabroisoflavanone A和B对3种主要蛋白(即1043、5XGI和6NJS)的对接分数在-6.5 ~ -9.0 kcal/mol之间。两种植物成分均表现出良好的抗结核敏感性评分。MD模拟(200 ns)进一步表明,光异黄酮A与5XGI形成了最稳定的配合物。Glabroisoflavanone a - 5xgi配合物结合最强,结合自由能最高(-75.19±4.89 kcal/mol),表明其相互作用强。这些发现突出了植物成分的差异结合和动态行为,为治疗开发提供了潜在的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


