Inclisiran for fast-track lipid-lowering treatment early after an acute coronary syndrome: a pilot study
IF 2.1
Q3 PERIPHERAL VASCULAR DISEASE
引用次数: 0
Abstract
Background
Elevated low-density lipoprotein cholesterol (LDL-C) after acute coronary syndrome (ACS) significantly increases cardiovascular risk. Timely reduction of LDL-C is crucial, but it takes several weeks to achieve optimal LDL-C levels with standard therapy. Monoclonal antibodies that inhibit PCSK9 have been demonstrated in some small randomised trials to rapidly abate LDL-C levels when used early after hospital admission for ACS. Inclisiran, a PCSK9-inhibiting siRNA, has recently been introduced into clinical practice; however, no information is available about its effectiveness and safety as a fast-track lipid-lowering agent in this clinical context.
Methods
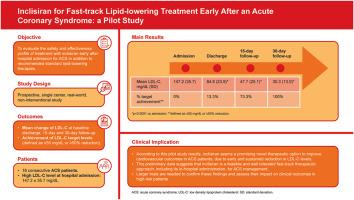
We conducted a prospective, real-world study evaluating a fast-track lipid-lowering approach starting inclisiran on top of standard therapy in 16 consecutive ACS patients admitted to our cardiac intensive care unit with a high baseline LDL-C level (147.2 ± 35.7 mg/dL). Patients started inclisiran as add-on therapy as soon as baseline LDL-C levels were available. We assessed LDL-C levels and the mean change of LDL-C at baseline, discharge, 15-day and 30-day follow-up.
Results
Inclisiran, added to standard therapy, reduced LDL-C levels to 30.3 ± 13.0 mg/dL at 30-day follow-up. The guideline-recommended LDL-C levels (≤55 mg/dL, ≥50 % reduction) were achieved in 73.3 % of patients at 15 days and in 100 % of patients at 30 days, with no adverse effects.
Conclusion
This pilot study shows promise for inclisiran as a novel therapeutic option to improve cardiovascular outcomes in patients with ACS by contributing to achieving an early and sustained reduction in LDL-C levels.
急性冠状动脉综合征后早期快速降脂治疗的Inclisiran:一项初步研究
背景:急性冠脉综合征(ACS)后低密度脂蛋白胆固醇(LDL-C)升高显著增加心血管风险。及时降低LDL-C是至关重要的,但标准治疗需要数周时间才能达到最佳LDL-C水平。在一些小型随机试验中,抑制PCSK9的单克隆抗体已被证明在因ACS入院后早期使用可迅速降低LDL-C水平。Inclisiran是一种抑制pcsk9的siRNA,最近已被引入临床实践;然而,在这种临床背景下,没有关于其作为快速降脂剂的有效性和安全性的信息。方法:我们进行了一项前瞻性、现实世界的研究,评估了在标准治疗基础上开始使用inclisiran的快速降脂方法,对16例连续入住我们心脏重症监护病房的高基线LDL-C水平(147.2±35.7 mg/dL)的ACS患者进行了评估。患者一旦获得基线LDL-C水平,就开始使用inclisiran作为附加治疗。我们评估LDL-C水平以及基线、出院、15天和30天随访时LDL-C的平均变化。结果在标准治疗中加入inclisiran后,随访30天LDL-C水平降至30.3±13.0 mg/dL。73.3%的患者在15天达到指南推荐的LDL-C水平(≤55 mg/dL,降低≥50%),100%的患者在30天达到指南推荐的LDL-C水平,无不良反应。本初步研究显示,inclisiran有望作为一种新的治疗选择,通过促进实现早期和持续降低LDL-C水平,改善ACS患者的心血管预后。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Atherosclerosis plus
Cardiology and Cardiovascular Medicine
CiteScore
2.60
自引率
0.00%
发文量
0
审稿时长
66 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


