Electrochemical strategy for scalable synthesis of MgO nanostructures for efficient removal of Malachite green
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
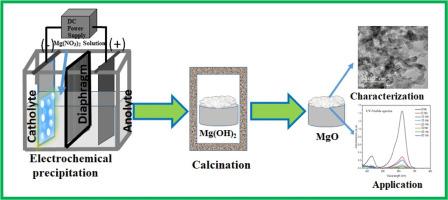
Present investigation demonstrates a simple and scalable electrochemical approach for synthesis of magnesium oxide (MO) nanoparticles, followed by their adoption on removal study of Malachite Green dye from aqueous solution. Magnesium hydroxide was electrochemically precipitated in a magnesium nitrate bath at a pH of 4.0, with a current density of 200 A m-². The resulting precipitated product was calcined for 2 hrs. at 500 °C to yield MgO nanoparticles (MO NPs). The MO NPs were characterized by X-ray diffraction (XRD) studies, RAMAN analysis, Fourier Transform Infrared spectroscopy (FTIR) and Field-emission scanning electron microscopy (FESEM) analysis, which confirmed the formation of pure-phase MgO nanoparticles with a flower-petal-like nanostructure. High-resolution transmission electron microscopy (HRTEM) analysis revealed the formation of hierarchical nanostructures with an average particle diameter of ≤ 50 nm. The mesoporous structure of MgO was well ascertained by nitrogen adsorption-desorption isotherms and was supported by pore size distribution analysis. A maximum adsorption capacity of 2000 mg g-1 was achieved under suitable conditions: 0.004 g of MgO in 20 mL of a 100 mg dm-3 Malachite green dye solution with a contact time of 45 min. Adsorption isotherm analysis revealed that the data fit both the Langmuir and Freundlich models; however, the Langmuir model exhibited better correlation, suggesting monolayer adsorption. Kinetic studies indicated that the adsorption followed a pseudo-second-order model, with intraparticle diffusion also playing a significant role in the overall adsorption mechanism. This study highlights an efficient and environmentally friendly method for synthesizing MgO-based adsorbents, offering strong potential for scalable industrial applications in water purification and dye removal.
可扩展合成高效去除孔雀石绿的MgO纳米结构的电化学策略
本研究展示了一种简单、可扩展的电化学合成氧化镁纳米颗粒的方法,并将其应用于孔雀石绿染料的去除研究。在pH为4.0,电流密度为200 a m-²的硝酸镁浴中,电化学沉淀氢氧化镁。将所得的沉淀产物煅烧2小时。在500°C下生成MgO纳米颗粒(MO NPs)。采用x射线衍射(XRD)、拉曼光谱(RAMAN)、傅里叶变换红外光谱(FTIR)和场发射扫描电镜(FESEM)对MO纳米粒子进行了表征,证实形成了具有花瓣状纳米结构的纯相MgO纳米粒子。高分辨率透射电镜(HRTEM)分析显示,形成了平均粒径≤50 nm的分层纳米结构。MgO的介孔结构通过氮吸附-解吸等温线得到了很好的确定,孔径分布分析也得到了支持。以100mg dm-3孔雀石绿染料溶液20ml为吸附剂,吸附剂浓度为0.004 g,接触时间为45min,最大吸附量为2000mg g-1。吸附等温线分析表明,数据符合Langmuir和Freundlich模型;然而,Langmuir模型显示出更好的相关性,表明单层吸附。动力学研究表明,吸附过程遵循准二阶模型,颗粒内扩散在整个吸附过程中也起着重要作用。该研究强调了一种高效且环保的合成mgo基吸附剂的方法,为水净化和染料去除的大规模工业应用提供了强大的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Surfaces and Interfaces
Chemistry-General Chemistry
CiteScore
8.50
自引率
6.50%
发文量
753
审稿时长
35 days
期刊介绍:
The aim of the journal is to provide a respectful outlet for ''sound science'' papers in all research areas on surfaces and interfaces. We define sound science papers as papers that describe new and well-executed research, but that do not necessarily provide brand new insights or are merely a description of research results.
Surfaces and Interfaces publishes research papers in all fields of surface science which may not always find the right home on first submission to our Elsevier sister journals (Applied Surface, Surface and Coatings Technology, Thin Solid Films)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


