A promising direct Z-scheme SnC/MoS2 heterojunction with superior optical absorption, high STH efficiency and strong catalytic activity for overall water-splitting
IF 3
Q2 PHYSICS, CONDENSED MATTER
引用次数: 0
Abstract
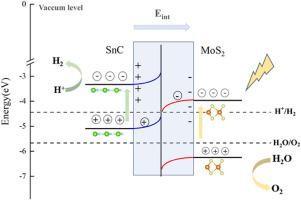
In this paper, we choose the SnC and MoS2 layers to construct heterojunction and investigate its geometrical stable, electrical property, charge transport, light absorption, solar-to-hydrogen energy conversion efficiency and photocatalytic performance using first-principles calculations. The results show that the SnC/MoS2 heterojunction is a semiconductor exhibiting a staggered (type-II) arrangement with an indirectly bandgap of 1.125 eV and good thermodynamic and thermal stabilities, which suppresses the photogenerated electron-hole pair recombination and significantly boosting the photocatalysis performance. The SnC/MoS2 heterojunction has a good band-edge positions to induce water decomposition, leading to the conduction-band reduction reaction on the SnC surface to generate H2 as well as the valence-band oxidation reaction on the MoS2 surface to produce O2. Furthermore, the SnC/MoS2 heterojunction has superior light absorbance compared with two single layers, showing the maximum absorbance peaks of 5.29 × 105 cm−1 in the visual light range, and higher solar-to-hydrogen energy conversion efficiency of 53.19 %. In addition, Gibbs free energy calculations show that the SnC/MoS2 heterojunction has high catalytic activity for redox reactions. All these show that the SnC/MoS2 heterojunction is a high efficiency direct Z-scheme heterojunction photocatalyst for over water-splitting.
一种有前途的直接Z-scheme SnC/MoS2异质结,具有优异的光吸收,高STH效率和强的催化活性
在本文中,我们选择SnC和MoS2层构建异质结,并使用第一性原理计算研究其几何稳定性,电学性质,电荷输运,光吸收,太阳能到氢的能量转换效率和光催化性能。结果表明,SnC/MoS2异质结是一种交错排列(ii型)的半导体,间接带隙为1.125 eV,具有良好的热力学和热稳定性,抑制了光生电子-空穴对复合,显著提高了光催化性能。SnC/MoS2异质结具有良好的能带边位置,可以诱导水分解,导致SnC表面发生导带还原反应生成H2, MoS2表面发生价带氧化反应生成O2。此外,SnC/MoS2异质结在可见光范围内的最大吸光度峰为5.29 × 105 cm−1,光能转换效率为53.19%。此外,Gibbs自由能计算表明SnC/MoS2异质结对氧化还原反应具有较高的催化活性。结果表明,SnC/MoS2异质结是一种高效的直接z型光催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


