Adsorptive removal of lead ions from wastewater using modified diatomite
IF 7.7
Q2 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
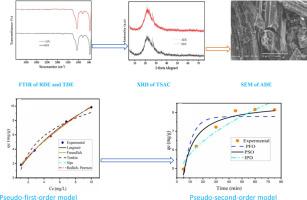
Adsorptive removal techniques offer a promising solution, and this study investigates the synthesis and application of sulfuric acid-modified diatomite for lead ion removal. Modified diatomite synthesized from raw diatomite was chemically modified and the activation parameters (acid concentration (2–10 M), activation time (4–12 h), solid-to-liquid ratio (50–250 g/L), and temperature (50–90 °C)) were optimized to obtain a 77.93 % lead ion removal efficiency under optimal conditions (4 M H₂SO₄, 10 h, 50 g/L, 90 °C). Then, it was characterized using scanning electron microscopy, Fourier transform infrared spectroscopy, Brunauer-Emmett-Teller analysis, and X-ray diffraction. Its structural transformation and surface area increment from 22.39–34.83 m²/g. We evaluated the adsorbent's adsorption efficiency under various experimental conditions, including solution pH (2–10), adsorbent dosage (0.2–1 g/100 mL), contact time (5–75 min), and initial lead ion concentration (10–50 ppm). Adsorption isotherms and kinetics were analyzed using the Langmuir, Freundlich, Sips, and Redlich-Peterson isotherms and pseudo-first-order, pseudo-second-order, and intraparticle diffusion kinetics models. The results demonstrated that modified diatomite effectively removed lead ions from aqueous solutions. Optimal adsorption conditions were determined at pH 7, 0.8 g/100 mL dosage, 45 min contact time, and 10 ppm initial lead ion concentration, yielding a maximum lead removal efficiency of 89.5 %. The adsorption process was well-described by the Langmuir and Sips isotherm models, the best-fit isotherm model, indicating the presence of a monolayer and heterogeneous surface interaction with a maximum adsorption capacity of 25.1 mg/g. Kinetic studies revealed that the intraparticle diffusion kinetics model best fit the kinetic model, suggesting that pore diffusion is the rate-limiting step in the interaction between lead ions and the adsorption site. This study shows that diatomite treated with sulfuric acid has the potential to be a viable and effective adsorbent for the removal of lead ions from wastewater, helping to provide sustainable water treatment solutions.
改性硅藻土吸附去除废水中的铅离子
吸附去除铅离子是一种很有前途的方法,本文研究了硫酸改性硅藻土的合成及其在铅离子去除中的应用。以原料硅藻土为原料合成改性硅藻土,并对活化参数(酸浓度(2 ~ 10 M)、活化时间(4 ~ 12 h)、料液比(50 ~ 250 g/L)、温度(50 ~ 90℃)进行了优化,在最佳条件(4 M h₂SO₄、10 h、50 g/L、90℃)下,铅离子去除率达到77.93%。然后用扫描电镜、傅里叶变换红外光谱、布鲁诺尔-埃米特-泰勒分析和x射线衍射对其进行了表征。结构变化和比表面积增加22.39 ~ 34.83 m²/g。我们在不同的实验条件下,包括溶液pH(2-10)、吸附剂用量(0.2-1 g/100 mL)、接触时间(5-75 min)和初始铅离子浓度(10-50 ppm),评估了吸附剂的吸附效率。采用Langmuir、Freundlich、Sips和Redlich-Peterson等温线、伪一阶、伪二阶和颗粒内扩散动力学模型分析了吸附等温线和动力学。结果表明,改性硅藻土能有效去除水溶液中的铅离子。最佳吸附条件为pH为7,用量为0.8 g/100 mL,接触时间为45 min,初始铅离子浓度为10 ppm,最大铅去除率为89.5%。Langmuir和Sips等温线模型(最适合的等温线模型)很好地描述了吸附过程,表明存在单层和非均相表面相互作用,最大吸附量为25.1 mg/g。动力学研究表明,颗粒内扩散动力学模型最符合动力学模型,表明孔隙扩散是铅离子与吸附位点相互作用的限速步骤。本研究表明,经硫酸处理的硅藻土有可能成为一种可行且有效的吸附废水中铅离子的吸附剂,有助于提供可持续的水处理解决方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of hazardous materials advances
Environmental Engineering
CiteScore
4.80
自引率
0.00%
发文量
0
审稿时长
50 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


