Polymeric resins with sulfonic or quaternary ammonium groups used as high-capacity adsorbents of tetracycline
IF 5.1
3区 工程技术
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
In this research, polymeric resins based on poly(3-sulfopropyl acrylate potassium salt) (P(SPAK)) and poly([3-(methacryloylamino)propyl] trimethylammonium chloride) (P(MAPTAC)) were prepared and tested as tetracycline (TC) adsorbents. These resins were characterized by chemical, thermal, morphological, surface techniques and by evaluating their hydration properties. P(SPAK) presented higher swelling ratio (27.6 g g−1) than P(MAPTAC) (15.6 g g−1). Also, we optimized by a multi-variate method the amount of resin and pH in TC removal using an experimental design, and then univariate evaluation of ionic strength concentration, contact time, TC concentration and temperature, reaching values of 713 mg g−1 for P(SPAK) at pH = 3 and 1067 mg g−1 for P(MAPTAC) at pH = 10.5, influenced by the chemical speciation of the pollutant and the resin. The low ionic strength favored adsorption, while high concentrations of KCl reduced it. The kinetics followed the Elovich model, reaching equilibrium in 1440 min. Freundlich isotherms indicated multilayer adsorption and energy heterogeneity. The process was endothermic and spontaneous with higher efficiency at higher temperatures. Finally, the resins proved to be reusable up to three consecutive adsorption-desorption cycles.
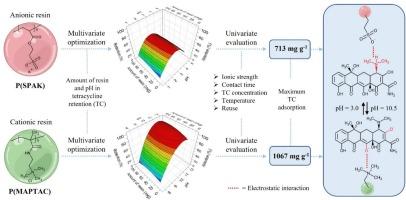
具有磺酸基或季铵基的聚合树脂,用作四环素的高容量吸附剂
本研究制备了以聚(3-(甲基丙烯酰胺)丙基]三甲基氯化铵)P(MAPTAC)和聚(3-(甲基丙烯酰胺)丙基)为基材的四环素(TC)吸附剂。通过化学、热、形态、表面技术和水合性能评价对树脂进行了表征。P(SPAK)的溶胀率(27.6 g g−1)高于P(MAPTAC) (15.6 g g−1)。此外,我们利用实验设计,通过多变量方法优化树脂用量和pH对TC去除的影响,然后对离子强度浓度、接触时间、TC浓度和温度进行单变量评估,在pH = 3时P(SPAK)的值为713 mg g−1,在pH = 10.5时P(MAPTAC)的值为1067 mg g−1,受污染物和树脂的化学形态的影响。低离子强度有利于吸附,而高浓度的KCl则降低了吸附。动力学遵循Elovich模型,在1440min达到平衡。Freundlich等温线显示多层吸附和能量非均质性。该过程是吸热自发的,在较高温度下效率较高。最后,树脂被证明可重复使用多达三个连续的吸附-解吸循环。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Reactive & Functional Polymers
工程技术-高分子科学
CiteScore
8.90
自引率
5.90%
发文量
259
审稿时长
27 days
期刊介绍:
Reactive & Functional Polymers provides a forum to disseminate original ideas, concepts and developments in the science and technology of polymers with functional groups, which impart specific chemical reactivity or physical, chemical, structural, biological, and pharmacological functionality. The scope covers organic polymers, acting for instance as reagents, catalysts, templates, ion-exchangers, selective sorbents, chelating or antimicrobial agents, drug carriers, sensors, membranes, and hydrogels. This also includes reactive cross-linkable prepolymers and high-performance thermosetting polymers, natural or degradable polymers, conducting polymers, and porous polymers.
Original research articles must contain thorough molecular and material characterization data on synthesis of the above polymers in combination with their applications. Applications include but are not limited to catalysis, water or effluent treatment, separations and recovery, electronics and information storage, energy conversion, encapsulation, or adhesion.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


