Biosorption selectivity of rare earth elements onto Euglena mutabilis suspensions and biofilms and the effect of divalent metal ions
IF 7.2
1区 化学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
The increasing demand for electronics has led to a desire to recover rare earth elements (REEs) from non-conventional sources, including mining and liquid waste effluents. Biosorption could be a promising method for adsorbing REEs onto microalgae, but biomass immobilization and light delivery challenges remain. It was recently shown that REEs biosorb 160% more on algal biofilms than suspended biomass due to the extracellular polymeric substance (EPS) matrix that grows abundantly in biofilms. In this work, we present findings on biosorption selectivity for different REEs in sulfate solutions. The maximum adsorption capacities of Euglena mutabilis suspensions and biofilms were determined for a mixed REE sulfate solution at an equimolar initial concentration range of 0.1–1 mol/L of each REE ion. The highest adsorption capacities for the suspension are for Sm and Eu which are 57% and 46% higher, respectively, compared to the average REE adsorption capacity. The biofilms also preferentially adsorb Sm, Eu, Yb and Lu at 0.035, 0.033, 0.033, and 0.031 mmol/g, respectively. The impact of dissolved divalent ions of Ca, Mg, and Fe on REE adsorption was also assessed. When Ca and Mg are added in equimolar amounts to 0.1–1 mmol/L solutions of equimolar La, Eu, and Yb sulfate, the amount of REEs adsorbed onto suspensions increases by 30% while when Fe is added, it decreases by 10%. No change is observed in biofilms except when Fe is added resulting in a reduction of the adsorption capacity by 40%. A possible explanation for the role of Fe is attributed to the formation of stronger bonds at the binding sites compared to Ca and Mg.
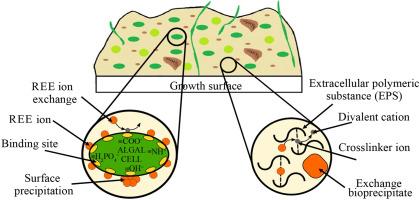
稀土元素在突变藻悬浮液和生物膜上的生物吸附选择性及二价金属离子的影响
对电子产品日益增长的需求导致人们希望从非常规来源(包括采矿和液体废水)中回收稀土元素(ree)。生物吸附是一种很有前途的将稀土吸附到微藻上的方法,但生物质固定化和光传递仍然存在挑战。最近的研究表明,由于细胞外聚合物(EPS)基质在生物膜中大量生长,藻类生物膜对稀土的生物吸收率比悬浮物高160%。在这项工作中,我们介绍了硫酸盐溶液中不同稀土元素的生物吸附选择性的研究结果。测定了异绿藻悬浮液和生物膜在初始等摩尔浓度范围为0.1 ~ 1 mol/L的稀土混合硫酸盐溶液中的最大吸附量。该悬浮液对钐和铕的吸附量最高,分别比REE平均吸附量高57%和46%。在0.035、0.033、0.033和0.031 mmol/g浓度下,生物膜对Sm、Eu、Yb和Lu具有较强的吸附能力。还评估了溶解的Ca、Mg和Fe二价离子对REE吸附的影响。当Ca和Mg以等摩尔量加入到0.1-1 mmol/L等摩尔的La、Eu和Yb硫酸盐溶液中时,稀土吸附量增加30%,而加入Fe时,稀土吸附量减少10%。在生物膜中没有观察到任何变化,除非添加铁导致吸附能力降低40%。对铁的作用的一种可能的解释是,与Ca和Mg相比,铁在结合位点形成了更强的键。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Rare Earths
化学-应用化学
CiteScore
8.70
自引率
14.30%
发文量
374
审稿时长
1.7 months
期刊介绍:
The Journal of Rare Earths reports studies on the 17 rare earth elements. It is a unique English-language learned journal that publishes works on various aspects of basic theory and applied science in the field of rare earths (RE). The journal accepts original high-quality original research papers and review articles with inventive content, and complete experimental data. It represents high academic standards and new progress in the RE field. Due to the advantage of abundant RE resources of China, the research on RE develops very actively, and papers on the latest progress in this field emerge every year. It is not only an important resource in which technicians publish and obtain their latest research results on RE, but also an important way of reflecting the updated progress in RE research field.
The Journal of Rare Earths covers all research and application of RE rare earths including spectroscopy, luminescence and phosphors, rare earth catalysis, magnetism and magnetic materials, advanced rare earth materials, RE chemistry & hydrometallurgy, RE metallography & pyrometallurgy, RE new materials, RE solid state physics & solid state chemistry, rare earth applications, RE analysis & test, RE geology & ore dressing, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


