Discovery of new dammarane saponins from Actinostemma lobatum: C-3 monoglucosylation governs α-glucosidase inhibition
IF 2.6
3区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Four new dammarane saponins, named actinostemmosides L-O (1–4), together with five known analogues (5–9), were isolated from the whole plant of Actinostemma lobatum. Their structures were established by HR-ESI-MS, exhaustive 1D/2D-NMR, and chemical methods. All nine saponins exhibited α-glucosidase inhibitory activity (IC50 40.3–150.9 μM), with actinostemmoside L (1) exhibiting an IC50 of 40.3 μM—twice the potency of acarbose (84.6 μM)—while 5 and 6 were equipotent with the drug. These results expand the chemical space of dammarane saponins and establish A. lobatum as a renewable source of α-glucosidase inhibitors.
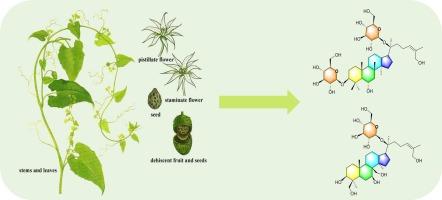
从放线素中发现新的达玛烷皂苷:C-3单糖基化控制α-葡萄糖苷酶抑制
从放线素茎(Actinostemma lobatum)全株中分离到4个新的达玛烷皂苷,命名为放线素茎苷L-O(1-4)和5个已知的类似物(5-9)。通过HR-ESI-MS、详尽的1D/2D-NMR和化学方法确定了它们的结构。9种皂苷均表现出α-葡萄糖苷酶抑制活性(IC50为40.3 μM ~ 150.9 μM),其中放线根皂苷L(1)的IC50为40.3 μM,是阿卡波糖(84.6 μM)的2倍,而5和6与阿卡波糖具有相同的抑制活性。这些结果扩大了达玛烷皂苷的化学空间,并确立了莲草作为α-葡萄糖苷酶抑制剂的可再生来源。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fitoterapia
医学-药学
CiteScore
5.80
自引率
2.90%
发文量
198
审稿时长
1.5 months
期刊介绍:
Fitoterapia is a Journal dedicated to medicinal plants and to bioactive natural products of plant origin. It publishes original contributions in seven major areas:
1. Characterization of active ingredients of medicinal plants
2. Development of standardization method for bioactive plant extracts and natural products
3. Identification of bioactivity in plant extracts
4. Identification of targets and mechanism of activity of plant extracts
5. Production and genomic characterization of medicinal plants biomass
6. Chemistry and biochemistry of bioactive natural products of plant origin
7. Critical reviews of the historical, clinical and legal status of medicinal plants, and accounts on topical issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


