DFT study on thermodynamic properties of liquid mixtures containing cyclohexanol with aniline and chloro-substituted anilines
IF 3.7
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
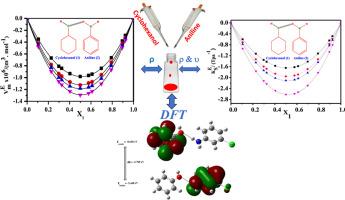
Density (ρ) and speed of sound (u) findings on the binary liquid mixtures consisting of cyclohexanol (CH—OH), with aniline (A), ortho-chloroaniline (o-CA), and meta-chloroaniline (m-CA) were gathered at the various temperatures spanning the entire concentration range. 303.15, 308.15,313.15 and 318.15 K at atmospheric pressure. The information measured there was utilized to compute excess molar volume (), excess isentropic compressibility (), excess of speed of sound (uE), excess intermolecular free length () and excess acoustic impedance (ZE). Further, the partial molar volumes (. . . ), partial molar compressibilities (, , , ) and their excess values (. . . ), (, , , ) were also computed to perceive more information on molecular interaction and structural effects in these mixtures. Applying the theory of Prigogine−Flory−Patterson (PFP) as a framework, the data of the current liquid mixtures were examined. The analysis of the experimental data took into consideration the interactions that occur between the individual molecules that make up liquid mixtures. By using density functional theory DFT (B3LYP) of 6-31 ++ G (d,P) to analyze the geometries, bond characteristics, interaction energies, and hydrogen bonded complexes in organic solvent phase, quantum chemical calculations were able to further confirm the hydrogen bonding that predominates between cyclohexanol with aniline and chlorosubstituted anilines.
环己醇与苯胺及氯代苯胺混合液体热力学性质的DFT研究
收集了环己醇(CH-OH)与苯胺(A)、邻氯苯胺(o-CA)和间氯苯胺(m-CA)二元液体混合物在整个浓度范围内不同温度下的密度(ρ)和声速(u)结果。303.15, 308.15,313.15和318.15 K在大气压下。利用测量到的信息计算了过量摩尔体积(VmE)、过量等熵压缩率(KSE)、过量声速(uE)、过量分子间自由长度(LfE)和过量声阻抗(ZE)。此外,偏摩尔体积(V¯m,1°。V¯ϕ1∘。∘V¯m 2。V¯ϕ,2°),部分摩尔可压缩性(Km,1°,K¯ϕ,1°,K¯m,2°,K¯ϕ,2°)及其余值(V¯m,1E。V¯ϕ1°E。V¯m 2 e。还计算了V¯ϕ,2°E), (K¯m,1E, K¯ϕ,1°E, K¯m,2E, K¯ϕ,2°E),以了解这些混合物中分子相互作用和结构效应的更多信息。以Prigogine - Flory - Patterson (PFP)理论为框架,对当前液体混合物的VmE数据进行了检验。对实验数据的分析考虑了构成液体混合物的单个分子之间发生的相互作用。利用6-31 ++ G (d,P)的密度泛函理论DFT (B3LYP)分析了有机溶剂相的几何形状、键特征、相互作用能和氢键配合物,量子化学计算进一步证实了环己醇与苯胺和氯取代苯胺之间以氢键为主。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chinese Journal of Chemical Engineering
工程技术-工程:化工
CiteScore
6.60
自引率
5.30%
发文量
4309
审稿时长
31 days
期刊介绍:
The Chinese Journal of Chemical Engineering (Monthly, started in 1982) is the official journal of the Chemical Industry and Engineering Society of China and published by the Chemical Industry Press Co. Ltd. The aim of the journal is to develop the international exchange of scientific and technical information in the field of chemical engineering. It publishes original research papers that cover the major advancements and achievements in chemical engineering in China as well as some articles from overseas contributors.
The topics of journal include chemical engineering, chemical technology, biochemical engineering, energy and environmental engineering and other relevant fields. Papers are published on the basis of their relevance to theoretical research, practical application or potential uses in the industry as Research Papers, Communications, Reviews and Perspectives. Prominent domestic and overseas chemical experts and scholars have been invited to form an International Advisory Board and the Editorial Committee. It enjoys recognition among Chinese academia and industry as a reliable source of information of what is going on in chemical engineering research, both domestic and abroad.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


