Constructing Co–O–Ce bridge in Mott-Schottky Co/CeO2 heterojunctions facilitates oxygen electrocatalysis bifunctionality for rechargeable Zn-air batteries
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
Abstract
Designing a heterogeneous interface to improve the kinetics of electrocatalysts represents an effective yet challenging approach for enhancing the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER). Herein, a simple MOF-assisted etching-pyrolysis strategy is proposed to fabricate an advanced Mott-Schottky (M–S) electrocatalyst composed of Co/CeO2 hetero-nanoparticles embedded within N-doped hollow carbon nanoboxes (H-Co/CeO2@NCBs). Notably, the interfacial Co–O–Ce bond bridging productively facilitates the electron transfer and modulates the charge distribution of the active center, thereby contributing to the ORR/OER kinetics. As expected, the optimal M–S H-Co/CeO2@NCBs catalyst exhibits promising bifunctional electrocatalytic activity with a small potential discrepancy of 0.65 V. Theoretical calculations reveal that the built-in electric field in the M–S heterojunction promotes electron transfer in oxygen electrocatalysis and the interfacial bridge-induced electron redistribution optimizes the adsorption/desorption of the oxygen intermediates, leading to reduced activation energy for the bifunctional ORR/OER reactions. Importantly, H-Co/CeO2@NCBs-assembled Zn-air battery (ZAB) delivers high power density (179.8 mW cm−2) and long-term stability (400 h). Furthermore, the assembled flexible solid-state ZAB with H-Co/CeO2@NCBs cathode also exhibits excellent charge–discharge reversibility and flexibility at various bending angles. This work provides a novel perspective on developing efficient and stable M–S bifunctional oxygen electrocatalysts.
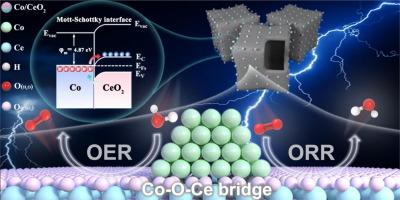
在Mott-Schottky Co/CeO2异质结中构建Co - o - ce桥促进了可充电锌空气电池的氧电催化双功能
设计非均相界面来改善电催化剂的动力学是提高氧还原反应(ORR)和析氧反应(OER)的有效但具有挑战性的方法。本文提出了一种简单的mof辅助蚀刻热解策略,制备了一种先进的Mott-Schottky (M-S)电催化剂,该催化剂由Co/CeO2异质纳米颗粒嵌入n掺杂的空心碳纳米盒(H-Co/CeO2@NCBs)中。值得注意的是,界面Co-O-Ce键桥接有效地促进了电子转移,调节了活性中心的电荷分布,从而促进了ORR/OER动力学。正如预期的那样,最佳的M-S H-Co/CeO2@NCBs催化剂具有良好的双功能电催化活性,电势差很小,为0.65 V。理论计算表明,M-S异质结内嵌的电场促进了氧电催化中的电子转移,界面桥诱导的电子重分配优化了氧中间体的吸附/解吸,导致双功能ORR/OER反应的活化能降低。重要的是,h - co /CeO2@NCBs-assembled锌空气电池(ZAB)具有高功率密度(179.8 mW cm−2)和长期稳定性(400 h)。此外,H-Co/CeO2@NCBs阴极组装的柔性固态ZAB在各种弯曲角度下也表现出良好的充放电可逆性和柔韧性。本研究为开发高效、稳定的M-S双功能氧电催化剂提供了新的思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


