Hinokitiol disrupts iron homeostasis and reduces virulence in Acinetobacter baumannii
IF 3.5
3区 医学
Q3 IMMUNOLOGY
引用次数: 0
Abstract
Background
Multidrug-resistant gram-negative bacterial infections pose a serious threat to human health. Given that iron levels affect bacterial pathogenesis, iron homeostasis is a promising target for antimicrobial therapies. We investigated the effects and mechanisms of action of hinokitiol against Acinetobacter baumannii, a notorious opportunistic pathogen that causes nosocomial infections worldwide.
Main methods
The minimum inhibitory concentration was determined using the broth microdilution method. The inhibition of A. baumannii isolates was determined by growth kinetics analysis and viable counts with or without iron supplementation following hinokitiol exposure. The expression status of acinetobactin-related genes and a motility-related gene was evaluated using RT-qPCR. The bioenergetics-related activity of hinokitiol-treated bacteria was assessed by determining the intracellular ATP levels and bacterial motility. The fluorescent probe 2ʹ,7′-dichlorodihydrofluorescein diacetate was used to detect intracellular reactive oxygen species.
Results
Hinokitiol decreased the number of viable bacteria in time- and concentration-dependent manners against A. baumannii. Hinokitiol-treated bacteria exhibited reduced intracellular iron levels and elevated expression of acinetobactin-related genes compared with untreated cells. Intracellular ATP levels, motility, and virulence-related characteristics were reduced; however, intracellular reactive oxygen species levels increased in A. baumannii following hinokitiol exposure. More survival nematodes were recorded for the Caenorhabditis elegans infection model using hinokitiol.
Significance
Hinokitiol affects bacterial iron homeostasis. Impaired bioenergetics-related activity and induced oxidative stress after hinokitiol treatment may contribute to the reduced viability of A. baumannii. Hinokitiol reduced bacterial adhesion, invasion, and cytotoxicity, which may be beneficial for treating A. baumannii infections.
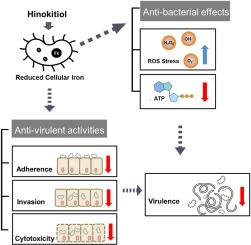
扁柏醇破坏鲍曼不动杆菌的铁稳态并降低毒力
耐多药革兰氏阴性细菌感染对人类健康构成严重威胁。考虑到铁水平影响细菌的发病机制,铁的体内平衡是抗微生物治疗的一个有希望的目标。我们研究了桧木醇对鲍曼不动杆菌的作用和机制,鲍曼不动杆菌是一种臭名昭著的机会致病菌,在世界范围内引起医院感染。主要方法采用微量肉汤稀释法测定最小抑菌浓度。通过生长动力学分析和在杉木醇暴露后添加或不添加铁的活菌计数来确定鲍曼不动杆菌的抑制作用。RT-qPCR检测不动杆菌素相关基因和运动相关基因的表达情况。通过测定细胞内ATP水平和细菌运动来评估扁柏醇处理细菌的生物能相关活性。用荧光探针2′,7′-二氯二氢荧光素检测细胞内活性氧。结果shinokitiol对鲍曼不动杆菌的抑菌效果呈时间依赖性和浓度依赖性。与未处理的细胞相比,扁柏醇处理的细菌表现出细胞内铁水平降低和不动杆菌素相关基因表达升高。细胞内ATP水平、运动性和毒力相关特征降低;然而,在扁柏醇暴露后,鲍曼不动杆菌的细胞内活性氧水平增加。用扁柏醇建立秀丽隐杆线虫感染模型,记录了更多的存活线虫。意义:扁柏醇影响细菌铁稳态。在扁柏醇处理后,生物能量相关活性受损和诱导氧化应激可能导致鲍曼不动杆菌活力降低。桧木醇可以降低细菌的粘附、侵袭和细胞毒性,这可能对治疗鲍曼不动杆菌感染有益。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microbial pathogenesis
医学-免疫学
CiteScore
7.40
自引率
2.60%
发文量
472
审稿时长
56 days
期刊介绍:
Microbial Pathogenesis publishes original contributions and reviews about the molecular and cellular mechanisms of infectious diseases. It covers microbiology, host-pathogen interaction and immunology related to infectious agents, including bacteria, fungi, viruses and protozoa. It also accepts papers in the field of clinical microbiology, with the exception of case reports.
Research Areas Include:
-Pathogenesis
-Virulence factors
-Host susceptibility or resistance
-Immune mechanisms
-Identification, cloning and sequencing of relevant genes
-Genetic studies
-Viruses, prokaryotic organisms and protozoa
-Microbiota
-Systems biology related to infectious diseases
-Targets for vaccine design (pre-clinical studies)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


