Dosage calculation model for humic acid-enhanced in-situ leaching of weathered crust elution-deposited rare earth ores
IF 5
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Humic acid (HA) is an environmentally friendly and efficient aid leaching agent, however, the mechanism of HA and ammonium sulfate ((NH4)2SO4) on rare earth (RE) in-situ leaching process and the effect of HA on ammonium reduction remain unclear. Herein, the potential of HA as an aid leaching agent to optimize the dosage of (NH4)2SO4 and reduce the residual ammonium salt was investigated, controlling the ammonia nitrogen pollution. Moreover, the calculation model of (NH4)2SO4 amount and unit consumption equation were established based on mass action law. The result indicated that the leaching efficiency of RE was increased by 3.96 % in the presence of 0.2 g·L−1 of HA, suggesting there was a synergistic effect between HA and (NH4)2SO4 during the RE leaching process. This effect was further confirmed by the leaching mechanism. Specifically, when the value of mass action quotient (Kt) increased, the reaction molar ratio (n) was decreased as compared with that of pure ammonium sulfate, indicating HA has a promoting effect on the ion exchange reaction between rare earth ions and ammonium ions. Additionally, it was revealed that the (NH4)2SO4 dosage was reduced by 34 % based on the law of mass action. Notably, the optimal (NH4)2SO4 concentration was lowered from 0.1 mol·L−1 for the traditional technology to 0.08 mol·L−1 for this work. These findings provided new insights into strengthening the leaching process of weathered crust elution-deposited rare earth ores by HA, which were valuable for elucidating the interaction mechanisms and realizing the source control of ammonia nitrogen pollution.
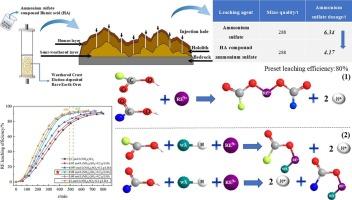
风化壳淋溶稀土矿腐植酸强化原位浸出用量计算模型
腐植酸(HA)是一种环保高效的助浸剂,但HA和硫酸铵(NH4)2SO4在稀土(RE)原位浸出过程中的作用机理以及HA对铵还原的影响尚不清楚。在此基础上,研究了腐殖酸作为辅助浸出剂在优化(NH4)2SO4投加量、减少铵盐残留量、控制氨氮污染方面的潜力。建立了基于质量作用定律的(NH4)2SO4用量计算模型和单位消耗方程。结果表明,当HA添加量为0.2 g·L−1时,稀土的浸出效率提高了3.96%,说明在稀土浸出过程中,HA与(NH4)2SO4之间存在协同作用。浸出机理进一步证实了这一效应。其中,随着质量作用商(Kt)的增大,反应摩尔比(n)较纯硫酸铵降低,说明HA对稀土离子与铵离子的离子交换反应有促进作用。此外,根据质量作用定律,发现(NH4)2SO4的用量减少了34%。最优(NH4)2SO4浓度从传统工艺的0.1 mol·L−1降低到0.08 mol·L−1。这些研究结果为强化风化壳淋溶稀土矿的HA浸出过程提供了新的认识,对阐明两者的相互作用机制和实现氨氮污染的源头控制具有重要意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Minerals Engineering
工程技术-工程:化工
CiteScore
8.70
自引率
18.80%
发文量
519
审稿时长
81 days
期刊介绍:
The purpose of the journal is to provide for the rapid publication of topical papers featuring the latest developments in the allied fields of mineral processing and extractive metallurgy. Its wide ranging coverage of research and practical (operating) topics includes physical separation methods, such as comminution, flotation concentration and dewatering, chemical methods such as bio-, hydro-, and electro-metallurgy, analytical techniques, process control, simulation and instrumentation, and mineralogical aspects of processing. Environmental issues, particularly those pertaining to sustainable development, will also be strongly covered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


