A multiplexed TSA/CRISPR-mediated one-pot system for rapid detection of high-risk animal-derived infectious diseases
IF 1.9
4区 生物学
Q4 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
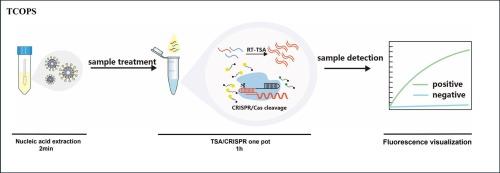
The importance of rapid and convenient pathogen detection has been emphasized by the alarming threat of the Coronavirus Disease 2019 (COVID-19) pandemic since 2019. Point-of-care testing (POCT) provides rapid diagnostic results directly at the sampling site. However, isothermal amplification-based POCT faces technical challenges including primer design complexity and false-positive rates. To address these limitations, we developed the Thermostatic Step Amplification (TSA)/Clustered regularly interspaced short palindromic repeats (CRISPR) One-Pot System (TCOPS). This sensitive, rapid, and efficient platform specifically detects Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Mpox virus (MPXV) and Rabíes virus (RV) through integrated amplification and CRISPR-based detection. Our integrated TCOPS overcomes the technical challenges through single-tube reactions combining thermostatic amplification and CRISPR detection, reducing contamination while maintaining high accuracy for field applications. TCOPS enables single-tube CRISPR detection of high-risk viruses, with 10 copies/μL sensitivity shown using cloned DNA template for RV. In evaluations against Quantitative Polymerase Chain Reaction (qPCR) using 50 clinical samples, TCOPS incorporating freeze-dried reagents and a newly developed miniature fluorescence system (Q max) demonstrated >90 % sensitivity and 100 % specificity. Combined with the portable Q max device and its lyophilized reagent kit, TCOPS enables simple, rapid detection of multiple zoonotic viruses (SARS-CoV-2, MPXV, and RV) at the point of care. This integrated system achieves high sensitivity and specificity while establishing a practical, field-deployable prototype for next-generation POCT applications in resource-limited settings.
一种多路TSA/ crispr介导的快速检测高风险动物源性传染病的单锅系统
2019年以来,2019冠状病毒病(COVID-19)大流行的惊人威胁凸显了快速便捷检测病原体的重要性。即时检测(POCT)直接在采样点提供快速诊断结果。然而,基于等温扩增的POCT面临着引物设计复杂性和假阳性率等技术挑战。为了解决这些限制,我们开发了恒温步进扩增(TSA)/聚集规律间隔短回文重复(CRISPR)一锅系统(TCOPS)。这个灵敏、快速、高效的平台通过集成扩增和基于crispr的检测,特异性检测严重急性呼吸综合征冠状病毒2 (SARS-CoV-2)、m痘病毒(MPXV)和Rabíes病毒(RV)。我们集成的TCOPS通过单管反应结合恒温扩增和CRISPR检测克服了技术挑战,减少了污染,同时保持了现场应用的高精度。TCOPS可以单管CRISPR检测高风险病毒,使用克隆的RV DNA模板显示灵敏度为10拷贝/μL。在使用50个临床样本进行定量聚合酶链反应(qPCR)的评估中,结合冻干试剂和新开发的微型荧光系统(qmax)的TCOPS显示出90%的灵敏度和100%的特异性。结合便携式Q max设备及其冻干试剂盒,TCOPS可以在护理点简单、快速地检测多种人畜共患病毒(SARS-CoV-2、MPXV和RV)。该集成系统具有高灵敏度和特异性,同时为资源有限的下一代POCT应用建立了实用的、可现场部署的原型。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of microbiological methods
生物-生化研究方法
CiteScore
4.30
自引率
4.50%
发文量
151
审稿时长
29 days
期刊介绍:
The Journal of Microbiological Methods publishes scholarly and original articles, notes and review articles. These articles must include novel and/or state-of-the-art methods, or significant improvements to existing methods. Novel and innovative applications of current methods that are validated and useful will also be published. JMM strives for scholarship, innovation and excellence. This demands scientific rigour, the best available methods and technologies, correctly replicated experiments/tests, the inclusion of proper controls, calibrations, and the correct statistical analysis. The presentation of the data must support the interpretation of the method/approach.
All aspects of microbiology are covered, except virology. These include agricultural microbiology, applied and environmental microbiology, bioassays, bioinformatics, biotechnology, biochemical microbiology, clinical microbiology, diagnostics, food monitoring and quality control microbiology, microbial genetics and genomics, geomicrobiology, microbiome methods regardless of habitat, high through-put sequencing methods and analysis, microbial pathogenesis and host responses, metabolomics, metagenomics, metaproteomics, microbial ecology and diversity, microbial physiology, microbial ultra-structure, microscopic and imaging methods, molecular microbiology, mycology, novel mathematical microbiology and modelling, parasitology, plant-microbe interactions, protein markers/profiles, proteomics, pyrosequencing, public health microbiology, radioisotopes applied to microbiology, robotics applied to microbiological methods,rumen microbiology, microbiological methods for space missions and extreme environments, sampling methods and samplers, soil and sediment microbiology, transcriptomics, veterinary microbiology, sero-diagnostics and typing/identification.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


