Cholesterol surface-modified oncolytic adenovirus enriched with apolipoprotein E penetrates the blood-brain barrier to target glioblastoma immunotherapy
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Glioblastoma (GBM) remains a therapeutic challenge due to its aggressive behaviour and the limitation of drug delivery by the blood-brain barrier (BBB). Conventional oncolytic adenoviruses (OAs) suffer from poor targeting efficiency. To overcome this limitation, we developed a cholesterol-modified OA (OA@Cho). This engineered virus actively regulates protein corona formation in the bloodstream, selectively enriching apolipoprotein E (ApoE). By exploiting low-density lipoprotein receptor (LDLR)-mediated BBB transcytosis, OA@Cho achieves precise glioma targeting and enhances therapeutic delivery. Critically, upon reaching the GBM site, OA@Cho induces an anti-tumor immune response, turning "cold" tumors into "hot" tumors by inducing immunogenic cell death (ICD). The proposed "surface modification-ApoE enrichment-receptor-mediated" paradigm establishes a transformative platform that enables oncolytic viruses to bypass biological barriers, thereby advancing targeted viral therapies against CNS malignancies with high translational relevance.
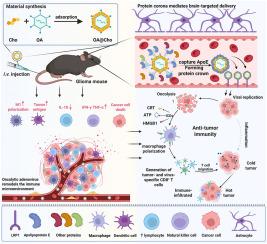
富含载脂蛋白E的胆固醇表面修饰溶瘤腺病毒穿透血脑屏障靶向胶质母细胞瘤免疫治疗
胶质母细胞瘤(GBM)由于其侵袭性行为和血脑屏障(BBB)给药的局限性,仍然是一个治疗挑战。传统的溶瘤腺病毒(OAs)靶向效率较差。为了克服这一限制,我们开发了一种胆固醇修饰的OA (OA@Cho)。这种工程病毒主动调节血液中蛋白冠的形成,选择性地富集载脂蛋白E (ApoE)。通过利用低密度脂蛋白受体(LDLR)介导的血脑屏障转胞作用,OA@Cho实现了精确的胶质瘤靶向并增强了治疗递送。关键的是,在到达GBM部位后,OA@Cho诱导抗肿瘤免疫反应,通过诱导免疫原性细胞死亡(ICD)将“冷”肿瘤转化为“热”肿瘤。提出的“表面修饰- apoe富集-受体介导”模式建立了一个转化平台,使溶瘤病毒能够绕过生物屏障,从而推进靶向治疗具有高翻译相关性的中枢神经系统恶性肿瘤的病毒治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


