Phosphorylation of plectin by Akt3 promotes triple-negative breast cancer cell invasive migration
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Triple-negative breast cancer (TNBC) lacks targeted therapeutics and is aggressive with a poor prognosis. The phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway, frequently deregulated in cancers, plays crucial roles in tumorigenesis and cancer progression. However, the distinct functions of the three Akt isoforms (Akt1, Akt2, Akt3) in these processes are not well understood. Here, we focus on Akt3, the least-studied Akt isoform, which is overexpressed in 28% of TNBC cases and significantly promotes TNBC growth, stemness, and epithelial-mesenchymal transition. Through a genome-wide proteomic screen, we identified plectin, a member of the plakin family, as an Akt3 substrate in TNBC cells. The depletion of plectin potently inhibits TNBC cell migration and invadopodia formation, albeit with mild effects on cell growth. The phosphorylation of plectin at Ser4268 by Akt promotes its colocalization with vimentin and TNBC cell migration. Our findings underscore the importance of Akt3-plectin signaling as a potential TNBC therapeutic target.
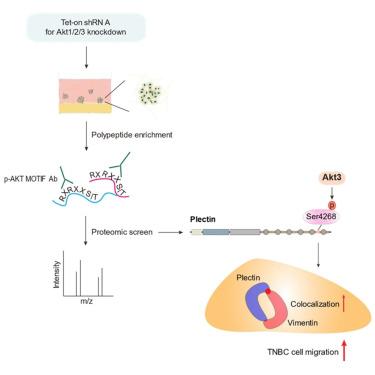
Akt3磷酸化plectin促进三阴性乳腺癌细胞侵袭性迁移
三阴性乳腺癌(TNBC)缺乏靶向治疗,具有侵袭性,预后差。磷脂酰肌醇3-激酶(PI3K)/Akt信号通路在癌症中经常失调,在肿瘤发生和癌症进展中起着至关重要的作用。然而,Akt三种亚型(Akt1, Akt2, Akt3)在这些过程中的不同功能尚不清楚。在这里,我们关注的是Akt亚型Akt3,这是研究最少的Akt亚型,它在28%的TNBC病例中过表达,并显著促进TNBC的生长、干性和上皮-间质转化。通过全基因组蛋白质组学筛选,我们发现plectin是plakin家族的一员,是TNBC细胞中Akt3的底物。尽管对细胞生长有轻微的影响,但plectin的缺失能有效地抑制TNBC细胞的迁移和侵足形成。Akt磷酸化plectin的Ser4268位点,促进其与vimentin和TNBC细胞迁移的共定位。我们的发现强调了Akt3-plectin信号作为潜在TNBC治疗靶点的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


