High-performance sodium-ion full cell using tunnel and layered-type composite cathode
IF 7.9
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Na0.44MnO2(NMO) powders are synthesized by auto combustion. At lower calcination temperature, carbothermal reductive environment preserves lamellar Birnessite type slab of NMO. A stress induced mechanism is proposed to explain the splitting of the lamellar nanosheet to NMO rods. TEM analyses confirm that the diffusion distance for Na+ ion extraction is much shorter than the growth direction to facilitate the extraction of Na+ from NMO lattice. Due to facile extraction of Na+ ions, remarkable 1st charge capacity ∼96 mAhg−1 is obtained in NMO cathode. Composite of NMO and TiO2 coated Na(Ni1/3Fe1/3Mn1/3)O2 (NFM) is made as a novel cathode for Na ion rechargeable cells. At 1C the discharge capacity of 0.7 NMO–0.3 NFM composite is reported to be 113 mAhg−1 with a capacity retention of ∼80 % after 100 cycles. The composite cathode also exhibits superior rate performance with a discharge capacity ∼66 mAhg−1 at 15C rate. In a voltage window of 1.0–4.0V, the discharge capacity of hard carbon and 0.7NMO – 0.3NFM full cell is measured to be 133 mAhg−1 and 109 mAhg−1 at 0.1 and 1C respectively. After 100 cycles, at 1C rate, we have reported 80 % capacity retention of the full cell.
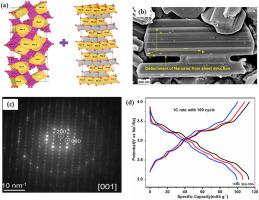
采用隧道和层状复合阴极的高性能钠离子电池
采用自燃烧法合成了Na0.44MnO2(NMO)粉体。在较低的煅烧温度下,碳热还原环境保留了NMO层状贝氏体型板坯。提出了一种应力诱导的机制来解释层状纳米片分裂成NMO棒。TEM分析证实,Na+离子萃取的扩散距离远短于生长方向,有利于Na+从NMO晶格中萃取。由于易于提取Na+离子,NMO阴极获得了显著的第一次电荷容量~ 96 mAhg−1。制备了NMO和TiO2包覆的Na(Ni1/3Fe1/3Mn1/3)O2 (NFM)复合材料作为Na离子可充电电池的新型阴极材料。据报道,在1C下,0.7 NMO-0.3 NFM复合材料的放电容量为113 mAhg−1,在100次循环后容量保持率为80%。复合阴极还表现出优异的倍率性能,在15C倍率下放电容量为~ 66 mAhg−1。在1.0-4.0V电压窗下,硬碳和0.7NMO - 0.3NFM全电池在0.1和1C下的放电容量分别为133 mAhg−1和109 mAhg−1。在1C倍率下,经过100次循环后,我们报告了80%的容量保留。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


