Gut virome characteristics associated with early onset of anemia and neurodevelopmental delay in preterm infants
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Early-onset anemia (EOA) and neurodevelopmental delay (NDD) are highly prevalent in preterm infants, causing substantial long-term health impacts. This study aimed to identify distinctive gut virome characteristics and their associations with EOA and NDD. We hypothesized that gut microbial colonization types and bacteriophage profiles may be risk factors for NDD in preterm infants with EOA. Fecal samples from 107 healthy preterm infants within the first week of life underwent virome and 16S rRNA sequencing. Consensus clustering of viral species signatures divided infants into four groups. The high EOA risk group showed significantly higher virome alpha diversity. Enriched Circoviridae sp. and uncultured Caudoviridae phage, along with reduced CRESS virus sp., were linked to elevated NDD risk. Geobacillus virus Tp84—the only bacteriophage exhibiting both temperate and virulent lifestyles—was associated with high EOA risk but low NDD risk. These findings highlight the role of gut virome in EOA and NDD pathogenesis, suggesting potential for targeted bacteriophage-based interventions to mitigate EOA-related NDD in preterm infants.
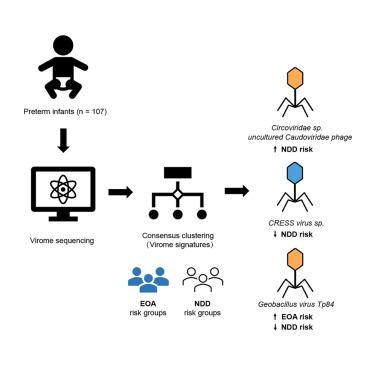
肠道病毒特征与早产儿早发性贫血和神经发育迟缓相关
早发性贫血(EOA)和神经发育迟缓(NDD)在早产儿中非常普遍,对健康造成重大的长期影响。本研究旨在确定独特的肠道病毒特征及其与EOA和NDD的关系。我们假设肠道微生物定植类型和噬菌体谱可能是EOA早产儿NDD的危险因素。对107名健康早产儿在出生后第一周内的粪便样本进行了病毒和16S rRNA测序。病毒种类特征的一致聚类将婴儿分为四组。高EOA风险组表现出更高的病毒α多样性。环状病毒科和未培养尾状病毒科噬菌体的富集,以及CRESS病毒科的减少,与NDD风险升高有关。土杆菌病毒tp84是唯一一种同时表现出温和和毒性生活方式的噬菌体,与高EOA风险相关,但与低NDD风险相关。这些发现强调了肠道病毒在EOA和NDD发病机制中的作用,提示基于靶向噬菌体的干预措施可能减轻早产儿EOA相关的NDD。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


