Urea-enhanced nucleation in the atmosphere: Mechanistic insights from MSA, SA, and ternary MSA-MA pathways
IF 3.7
2区 环境科学与生态学
Q2 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
New particle formation (NPF) is a fundamental process governing atmospheric aerosol production and, consequently, climate and air quality. Despite the extensive study of acid-base interactions in nucleation, the role of organic nitrogen compounds, particularly urea, remains inadequately explored. Here, we employ quantum chemical calculations and kinetic simulations via the Atmospheric Cluster Dynamics Code (ACDC) to elucidate the mechanisms by which urea modulates nucleation processes. Our investigation reveals that urea, through its dual hydrogen-bonding capability and proton-accepting -C=O group, stabilizes clusters formed with methanesulfonic acid (MSA), sulfuric acid (SA), and nitric acid (NA). In binary systems, MSA-Urea clusters exhibit intermediate stability, higher than those of MSA-NH3 but lower than MSA-MA. Notably, in ternary MSA-MA-Urea systems, urea markedly enhances nucleation rates, particularly under low-temperature conditions. These results indicate that urea can compensate for the low ambient concentrations of more potent bases such as methylamine (MA), thus playing a pivotal role in atmospheric nucleation. Our findings underscore the need to incorporate organic nitrogen chemistry into atmospheric models to improve predictions of aerosol formation and its broader climatic impacts.
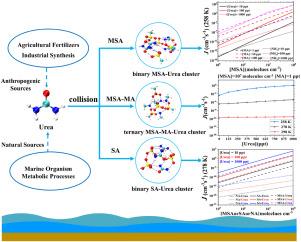
尿素在大气中增强成核:从MSA, SA和三元MSA- ma途径的机制见解
新粒子形成(NPF)是控制大气气溶胶产生的基本过程,因此也控制着气候和空气质量。尽管对酸碱相互作用在成核中的作用进行了广泛的研究,但有机氮化合物,特别是尿素的作用仍然没有得到充分的探讨。在这里,我们采用量子化学计算和通过大气簇动力学代码(ACDC)的动力学模拟来阐明尿素调节成核过程的机制。我们的研究表明,尿素通过其双氢键能力和质子接受-C=O基团,稳定了由甲磺酸(MSA)、硫酸(SA)和硝酸(NA)形成的团簇。在二元体系中,msa -尿素团簇表现出中等稳定性,高于MSA-NH3,但低于MSA-MA。值得注意的是,在三元msa - ma -尿素体系中,尿素显著提高了成核速率,特别是在低温条件下。这些结果表明,尿素可以补偿低浓度的环境中更有效的碱,如甲胺(MA),从而在大气成核中起关键作用。我们的发现强调了将有机氮化学纳入大气模型以改进气溶胶形成及其更广泛的气候影响的预测的必要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Atmospheric Environment
环境科学-环境科学
CiteScore
9.40
自引率
8.00%
发文量
458
审稿时长
53 days
期刊介绍:
Atmospheric Environment has an open access mirror journal Atmospheric Environment: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Atmospheric Environment is the international journal for scientists in different disciplines related to atmospheric composition and its impacts. The journal publishes scientific articles with atmospheric relevance of emissions and depositions of gaseous and particulate compounds, chemical processes and physical effects in the atmosphere, as well as impacts of the changing atmospheric composition on human health, air quality, climate change, and ecosystems.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


