Galactan side chains dominate thermal aggregation of RGI-Rich pectin during drying resulting poor solubility
IF 12.5
1区 化学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Rhamnogalacturonan I (RG-I) pectin, abundant in fruit and vegetable waste, exhibits great potential for applications in the food and cosmetics industries. However, RGI-rich pectin tends to aggregate during heat-drying, leading to poor solubility, which severely restricts its utilization. This study delves into the role of RGI side chain structures in aggregation. Different RGI-rich pectins were extracted from citrus and potato and modified enzymatically. The results showed that the solubility of the hydrolyzed pectins (arabinose or galactose side chain shortened to about 50 % of their original length, respectively) was significantly improved, even more than doubled for citrus RGI-rich pectin. Galactose side chain removal showed a more pronounced effect. Meanwhile, the soluble and insoluble fractions were separated, and their structural characteristics were determined. The results showed that the insoluble fraction was found to have a higher length of arabinose and galactose side chains, with greater branching, which contributed to tighter molecular conformations. Additionally, the introduction of urea to disrupt inter-molecular hydrogen bonding significantly enhanced solubility, emphasizing that hydrogen bonds mediate entanglement of RG-I side chains. These findings provide new insights into the thermal aggregation mechanism of RGI-rich pectin, paving the way for strategies to boost its solubility and industrial application performance.
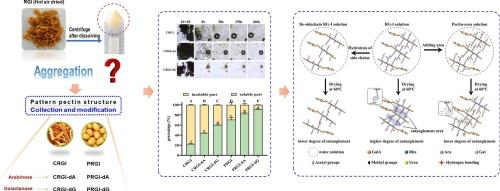
在干燥过程中,半乳聚糖侧链主导富含rgi的果胶的热聚集,导致溶解度差
鼠李糖半乳素I (RG-I)果胶是一种富含果蔬废弃物的果胶,在食品和化妆品工业中具有广阔的应用前景。然而,富含rgi的果胶在热干燥过程中容易聚集,导致其溶解度差,严重制约了其利用。本研究探讨了RGI侧链结构在聚合中的作用。从柑橘和马铃薯中提取不同的富含rgi的果胶,并进行酶修饰。结果表明,水解果胶的溶解度(阿拉伯糖或半乳糖侧链分别缩短至其原始长度的50%左右)显著提高,柑橘富含rgi果胶的溶解度甚至增加了一倍以上。半乳糖侧链去除效果更明显。同时,分离了可溶性和不溶性组分,测定了它们的结构特征。结果表明,不溶性组分具有更长的阿拉伯糖和半乳糖侧链,具有更大的分支,这有助于更紧密的分子构象。此外,引入尿素破坏分子间氢键显著提高了溶解度,强调氢键介导RG-I侧链的缠结。这些发现为了解富含rgi果胶的热聚集机制提供了新的见解,为提高其溶解度和工业应用性能的策略铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbohydrate Polymers
化学-高分子科学
CiteScore
22.40
自引率
8.00%
发文量
1286
审稿时长
47 days
期刊介绍:
Carbohydrate Polymers stands as a prominent journal in the glycoscience field, dedicated to exploring and harnessing the potential of polysaccharides with applications spanning bioenergy, bioplastics, biomaterials, biorefining, chemistry, drug delivery, food, health, nanotechnology, packaging, paper, pharmaceuticals, medicine, oil recovery, textiles, tissue engineering, wood, and various aspects of glycoscience.
The journal emphasizes the central role of well-characterized carbohydrate polymers, highlighting their significance as the primary focus rather than a peripheral topic. Each paper must prominently feature at least one named carbohydrate polymer, evident in both citation and title, with a commitment to innovative research that advances scientific knowledge.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


