Activated biochar from Co-hydrothermal carbonization of municipal sludge and enteromorpha for high-efficiency Pb(II) removal: Activation optimization and adsorption mechanism
IF 5.8
2区 生物学
Q1 AGRICULTURAL ENGINEERING
引用次数: 0
Abstract
The increasing accumulation of municipal sludge poses significant environmental challenges. Hydrothermal carbonization (HTC), as an advanced technology for the harmless treatment and resource recovery of sludge, enables the efficient conversion of organic matter into biochar. However, the resulting biochar is severely limited in its potential as an effective adsorbent due to its low carbon content and high ash content. This work addressed this limitation through co-hydrothermal carbonization of municipal sludge with Enteromorpha, a high-carbon, low-ash biomass waste. Activated biochar derived from the hydrothermal biochar was prepared by using KOH activation for Pb(II) adsorption in aqueous solutions. The effects of activation conditions on biochar yield and adsorption capacity were systematically investigated, along with the influence of adsorption parameters and the underlying mechanisms of Pb(II) removal. Optimal activation was achieved at 600 °C using 2 mol L−1 KOH for 60 min, resulting in an adsorption capacity of 48.6 mg g−1 and a biochar yield of 54.7 %. Adsorption kinetics analysis indicates that the process of active biochar adsorbing Pb(II) involves multiple mechanism interactions, including physical adsorption, complexation, electrostatic attraction, ion exchange, precipitation, and cation-π interactions.
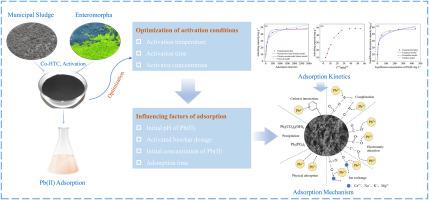
城市污泥和enteromorpha共水热炭化活性生物炭高效去除Pb(II):活化优化及吸附机理
城市污泥的日益积累对环境构成了重大挑战。水热炭化(HTC)是一种先进的污泥无害化处理和资源化利用技术,可以将有机物高效转化为生物炭。然而,由此产生的生物炭由于其低碳含量和高灰分含量而严重限制了其作为有效吸附剂的潜力。本研究通过利用Enteromorpha(一种高碳、低灰分的生物质废弃物)对城市污泥进行共水热碳化,解决了这一问题。以水热生物炭为原料,采用KOH活化法制备了吸附Pb(II)的活性生物炭。系统研究了活化条件对生物炭产率和吸附量的影响、吸附参数的影响以及去除Pb(II)的机理。在600℃条件下,用2 mol L−1 KOH活化60 min,吸附量为48.6 mg g−1,生物炭收率为54.7%。吸附动力学分析表明,活性生物炭吸附Pb(II)的过程涉及物理吸附、络合、静电吸引、离子交换、沉淀和阳离子-π相互作用等多种机理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biomass & Bioenergy
工程技术-能源与燃料
CiteScore
11.50
自引率
3.30%
发文量
258
审稿时长
60 days
期刊介绍:
Biomass & Bioenergy is an international journal publishing original research papers and short communications, review articles and case studies on biological resources, chemical and biological processes, and biomass products for new renewable sources of energy and materials.
The scope of the journal extends to the environmental, management and economic aspects of biomass and bioenergy.
Key areas covered by the journal:
• Biomass: sources, energy crop production processes, genetic improvements, composition. Please note that research on these biomass subjects must be linked directly to bioenergy generation.
• Biological Residues: residues/rests from agricultural production, forestry and plantations (palm, sugar etc), processing industries, and municipal sources (MSW). Papers on the use of biomass residues through innovative processes/technological novelty and/or consideration of feedstock/system sustainability (or unsustainability) are welcomed. However waste treatment processes and pollution control or mitigation which are only tangentially related to bioenergy are not in the scope of the journal, as they are more suited to publications in the environmental arena. Papers that describe conventional waste streams (ie well described in existing literature) that do not empirically address ''new'' added value from the process are not suitable for submission to the journal.
• Bioenergy Processes: fermentations, thermochemical conversions, liquid and gaseous fuels, and petrochemical substitutes
• Bioenergy Utilization: direct combustion, gasification, electricity production, chemical processes, and by-product remediation
• Biomass and the Environment: carbon cycle, the net energy efficiency of bioenergy systems, assessment of sustainability, and biodiversity issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


