Glycyrrhizic acid-based nanotheranostic system to enhance photothermal immunotherapy by suppressing HSP90 and remodeling tumor microenvironment
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Anticancer photothermal therapy efficacy is currently restricted by heat shock protein (HSP90)-mediated thermoresistance, immunosuppressive microenvironment, and poor delivery efficiency of photosensitizers. At present, the “all-in-one” therapeutic system that can simultaneously achieve tumor-targeted delivery, efficient photothermal therapy, and tumor immune enhancement effects is desperately needed. We previously fabricated Glycyrrhizic acid (GL)-based lipid nanoparticles (GLPs) that possess hepatocellular carcinoma (HCC) targeting and improved membrane stability. Interestingly, GL exhibits the potential to inhibit the up-regulated HSP90 and regulate the immunosuppressive tumor microenvironment. Herein, the near-infrared photosensitizer IR780 was efficiently encapsulated into GLPs to promote photothermal enhancement and synergistic immunosuppressive anti-tumor effects. Under the irradiation of NIR light, the IR780 GLPs effectively enhance the PTT efficacy on the HCC-bearing mice model via suppressing HSP90 expression with the combination of GL. Furthermore, IR780 GLPs exhibited strong HCC targeting ability, effectively downregulating the expression of immunosuppressive Treg cells, promoting the repolarization of M2 macrophages to M1 macrophages, increasing the infiltration of cytotoxic CD8+T cells and downregulating the expression of TGF-β and IL-10, upregulating IL-12, TNF-α, and IFN-γ, reshaping the tumor immunosuppressive microenvironment. Overall, we developed a novel GL-based lipid nanoparticle system encapsulating IR780 to amplify the synergistic effects of chemo-PTT for HCC treatment.
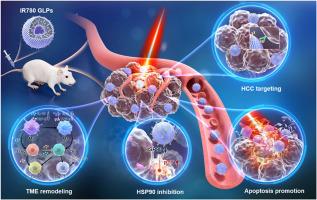
甘草酸纳米治疗系统通过抑制HSP90和重塑肿瘤微环境来增强光热免疫治疗
目前,肿瘤光热治疗的效果受到热休克蛋白(HSP90)介导的热阻、免疫抑制微环境和光敏剂递送效率差等因素的限制。目前迫切需要一种能够同时实现肿瘤靶向递送、高效光热治疗和肿瘤免疫增强效果的“一体化”治疗系统。我们之前制造了甘草酸(GL)为基础的脂质纳米颗粒(GLPs),具有靶向肝细胞癌(HCC)和提高膜稳定性。有趣的是,GL显示出抑制上调的HSP90和调节免疫抑制肿瘤微环境的潜力。本研究将近红外光敏剂IR780有效地包裹在glp中,促进光热增强和协同免疫抑制抗肿瘤作用。在近红外光照射下,IR780 GLPs通过联合GL抑制HSP90的表达,有效增强了肝癌小鼠模型的PTT疗效。此外,IR780 GLPs表现出较强的HCC靶向能力,可有效下调免疫抑制Treg细胞的表达,促进M2巨噬细胞向M1巨噬细胞的再极化,增加细胞毒性CD8+T细胞的浸润,下调TGF-β和IL-10的表达。上调IL-12、TNF-α和IFN-γ,重塑肿瘤免疫抑制微环境。总的来说,我们开发了一种新型的基于gl的脂质纳米颗粒系统,包封IR780,以增强化疗- ptt对HCC治疗的协同作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


