Integration of lymphatic vasculature to a human lymph node-on-chip enhances physiological immune properties
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
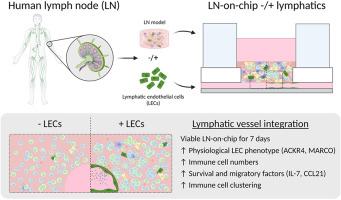
To study systemic human innate and adaptive immune responses in detail, competent in vitro lymph node (LN) models with LN stromal cells (LNSCs) are required to recapitulate the physiological microenvironment. The multicellular organisation of LNs possesses a challenge for designing such microphysiological systems (MPS), particularly with the structural complexity of LNs and the lymphatic vasculature. Here, we established an organotypic LN model with integrated lymphatics in an organ-on-chip (OoC) platform containing a printed sacrificial structure, and studied the influence of a perfused lymphatic endothelial cell (LEC)-lined channel on the LN-on-chip microenvironment. Upon one-week of culture under lymphatic flow, LECs lined the tubular structure forming a lymphatic vessel through the LN model, and stable metabolic conditions within the LN-on-chip were confirmed. Interestingly, LECs in the LN-on-chip displayed the phenotype found in human LNs with upregulation of LEC-specific LN markers, such as atypical chemokine receptor 4 (ACKR4). The presence of the LEC-lined perfused vessel in the LN-on-chip resulted in the increase of native immune cells, most notably B cells, and the secretion of survival and migratory signals, namely interleukin-7 (IL-7) and CC motif chemokine ligand 21 (CCL21). Likewise, LECs promoted the abundance of immune cell clusters closer to the vessel. As such, these features represent an enhanced physiological microenvironment to allow for immune cell migration and interactions for efficient LN functioning. This approach paves the way for LN integration into multi-OoC (MOC) platforms to investigate immunological crosstalk between tissue-derived factors, immune cell trafficking and organ-specific adaptive immune responses.
将淋巴管系统整合到人体淋巴结芯片上,增强生理免疫特性
为了详细研究人类系统性先天免疫和适应性免疫反应,需要具有LN基质细胞(LNSCs)的体外淋巴结(LN)模型来重现生理微环境。LNs的多细胞组织对设计这样的微生理系统(MPS)具有挑战,特别是LNs和淋巴血管的结构复杂性。在此,我们在包含打印牺牲结构的器官片上(OoC)平台上建立了集成淋巴管的器官型LN模型,并研究了淋巴内皮细胞(LEC)灌注通道对LN片上微环境的影响。淋巴流培养一周后,通过LN模型,LECs排列在管状结构上形成淋巴管,证实了LN芯片内稳定的代谢条件。有趣的是,芯片上LN中的LECs表现出在人类LN中发现的表型,即LECs特异性LN标记上调,如非典型趋化因子受体4 (ACKR4)。在芯片上的ln中,有了lec衬里的灌注血管,导致了天然免疫细胞(尤其是B细胞)的增加,以及生存和迁移信号,即白细胞介素-7 (IL-7)和CC基序趋化因子配体21 (CCL21)的分泌。同样,LECs促进了靠近血管的免疫细胞簇的丰度。因此,这些特征代表了一个增强的生理微环境,允许免疫细胞迁移和有效LN功能的相互作用。该方法为LN整合到多ooc (MOC)平台以研究组织源性因子、免疫细胞运输和器官特异性适应性免疫应答之间的免疫串话铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


