Lipid nanoparticle-mediated mRNA/siRNA dual-bioengineered dendritic cell vaccines combined of PD-1/PD-L1 blockade for boosting tumor immunotherapy
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
DC vaccines occupy a pivotal position in the realm of cancer treatment, leveraging the immune system to precisely target and effectively eliminate tumor cells. However, current challenges in tumor DC vaccine development include issues such as limited efficacy and potential upregulation of immunosuppressive molecules like PD-L1 on tumor cells. Herein, we employed microfluidic technology to fabricate lipid nanoparticles (LNPs) that simultaneously encapsulate OVA mRNA (mOVA), encoding the model antigen OVA, and siRNA targeting PD-L1. The LNP/mOVA/siPD-L1 nanoparticles were utilized to transfect bone marrow-derived dendritic cells (BMDCs) to construct dual-bioengineered DC vaccines. Subsequently, the efficacy of the dually bioengineered DC vaccines was verified in both prophylactic and therapeutic tumor vaccine models. The upregulation of PD-L1 protein expression on the surface of tumor cells was further blocked by combination of anti-PD-L1 antibody therapy. Notably, the combined therapy achieved complete tumor suppression and the rechallenge experiments demonstrated the tumor immune memory effect induced by the combined therapy, highlighting its potential to elicit long-lasting immunity against cancer. Overall, our findings suggest that this combined therapy holds significant promise for the treatment, metastasis prevention, and recurrence management of tumors, with potential clinical application value in the future.
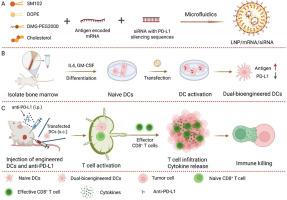
脂质纳米颗粒介导的mRNA/siRNA双生物工程树突状细胞疫苗联合PD-1/PD-L1阻断促进肿瘤免疫治疗
DC疫苗在癌症治疗领域占有举足轻重的地位,利用免疫系统精确靶向并有效消灭肿瘤细胞。然而,目前肿瘤DC疫苗开发面临的挑战包括诸如PD-L1等免疫抑制分子对肿瘤细胞的有限疗效和潜在上调等问题。在此,我们采用微流体技术制备脂质纳米颗粒(LNPs),同时封装OVA mRNA (mOVA),编码模型抗原OVA和靶向PD-L1的siRNA。利用LNP/mOVA/siPD-L1纳米颗粒转染骨髓源性树突状细胞(bmdc)构建双生物工程DC疫苗。随后,在预防性和治疗性肿瘤疫苗模型中验证了双生物工程DC疫苗的有效性。联合抗PD-L1抗体治疗进一步阻断肿瘤细胞表面PD-L1蛋白表达上调。值得注意的是,联合治疗实现了完全的肿瘤抑制,再挑战实验证明了联合治疗诱导的肿瘤免疫记忆效应,突出了其对癌症产生持久免疫的潜力。总之,我们的研究结果表明,这种联合治疗在肿瘤的治疗、预防转移和复发管理方面具有重要的前景,未来具有潜在的临床应用价值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


