Validation and sample bioanalysis of protoporphyrin IX in rat plasma by the surrogate matrix approach
IF 2.8
3区 医学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
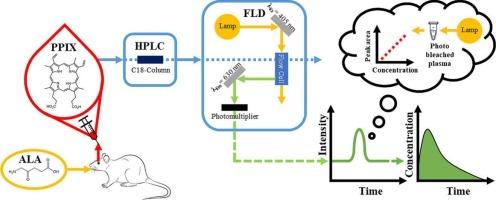
Protoporphyrin IX (PPIX) plays a pivotal role in the heme biosynthesis pathway and serves as both a valuable biomarker in clinical diagnostics and a photosensitizer in photodynamic applications. Despite its physiological importance, accurate quantification of endogenous PPIX in biological matrices remains challenging due to the lack of an analyte-free authentic control matrix and inherent baseline variability. This study describes the development and validation of a high-performance liquid chromatography method with fluorescence detection (HPLC-FLD) for PPIX quantification in rat plasma, applying a surrogate matrix strategy in full compliance with the International Council for Harmonisation M10 guideline.
Endogenous PPIX was removed from rat plasma by visible light-induced analyte stripping, enabling the in-house preparation of a surrogate matrix, for accurate calibration sample generation. The method showed appropriate selectivity, excellent linearity over the range of 10 and 700 ng/mL (r ≥ 0.995), satisfactory precision and accuracy across all validation levels. The lower limit of quantification was established at 10 ng/mL. The stability of both the analyte and internal standard was confirmed under various conditions, including 3 freeze–thaw cycles, short- and long-term storage, and autosampler residence. The method was successfully applied in an in vivo study in which male rats were treated with aminolevulinic acid to induce PPIX formation, thereby confirming its suitability for study sample analysis.
This fluorescence-based HPLC method offers a practical and cost-effective solution for monitoring PPIX in plasma samples. The bioanalytical method validation using a surrogate matrix approach for endogenous PPIX quantification fully aligned with current international regulatory standards may set a precedent for future method development in this field.
用替代基质法验证大鼠血浆中原卟啉IX的有效性及样品生物分析
原卟啉IX (PPIX)在血红素生物合成途径中起着关键作用,在临床诊断中是一种有价值的生物标志物,在光动力学应用中是一种光敏剂。尽管其在生理上具有重要意义,但由于缺乏无分析物的真实对照基质和固有的基线可变性,对生物基质中内源性PPIX的准确定量仍然具有挑战性。本研究描述了一种用于大鼠血浆中PPIX定量的高效液相色谱荧光检测(HPLC-FLD)方法的开发和验证,该方法采用替代基质策略,完全符合国际协调理事会M10指南。内源性PPIX通过可见光诱导的分析物剥离从大鼠血浆中去除,使替代基质的内部制备成为可能,以准确校准样品的生成。该方法在10 ~ 700 ng/mL范围内具有良好的选择性和良好的线性关系(r≥0.995),在所有验证水平上具有良好的精密度和准确度。定量下限为10 ng/mL。分析物和内标物在3次冻融循环、短期和长期储存、自动进样器停留等条件下的稳定性得到了证实。该方法已成功应用于雄性大鼠氨基乙酰丙酸诱导PPIX形成的体内研究,从而证实了该方法适用于研究样本分析。这种基于荧光的高效液相色谱方法为监测血浆样品中的PPIX提供了一种实用且具有成本效益的解决方案。使用替代矩阵方法进行内源性PPIX定量的生物分析方法验证完全符合当前的国际监管标准,可能为该领域未来的方法开发开创先例。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chromatography B
医学-分析化学
CiteScore
5.60
自引率
3.30%
发文量
306
审稿时长
44 days
期刊介绍:
The Journal of Chromatography B publishes papers on developments in separation science relevant to biology and biomedical research including both fundamental advances and applications. Analytical techniques which may be considered include the various facets of chromatography, electrophoresis and related methods, affinity and immunoaffinity-based methodologies, hyphenated and other multi-dimensional techniques, and microanalytical approaches. The journal also considers articles reporting developments in sample preparation, detection techniques including mass spectrometry, and data handling and analysis.
Developments related to preparative separations for the isolation and purification of components of biological systems may be published, including chromatographic and electrophoretic methods, affinity separations, field flow fractionation and other preparative approaches.
Applications to the analysis of biological systems and samples will be considered when the analytical science contains a significant element of novelty, e.g. a new approach to the separation of a compound, novel combination of analytical techniques, or significantly improved analytical performance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


