Uncovering pH-driven metastable transitions and thermal aggregation of HMW-GSs via all-atom molecular dynamics simulations
IF 11
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
High-molecular-weight glutenin subunits (HMW-GSs) are key contributors to wheat dough viscoelasticity, with their conformational adaptability playing a vital role in gluten network formation. In this study, we employed a two-stage molecular dynamics simulation approach to investigate how acidic (pH 2) and alkaline (pH 12) conditions modulate the metastable states of three x-type HMW-GSs-Ax1, Bx7, and Dx2-and how these conformations respond to thermal aggregation. Results revealed clear subunit-specific differences in conformational plasticity. Under extreme pH conditions, Ax1 and Bx7 displayed greater RMSD fluctuations and broader radius of gyration distributions compared to Dx2, demonstrating enhanced structural flexibility. Hydrogen bond lifetime analysis indicated that Bx7 maintained longer-lived interactions across metastable states, implying greater internal stabilization. Upon thermal acceleration at 400 K, Ax1 rapidly progressed toward aggregation-prone conformations, whereas Dx2 preserved partial structural integrity, reflecting its superior thermal resilience. Covariance-based cross-correlation analysis further showed extensive domain decoupling in Ax1 and Bx7, in contrast to the more coordinated dynamics of Dx2. By linking metastable ensembles to aggregation behavior, this study provides mechanistic insights into gluten protein adaptability. These molecular insights may also serve as a reference for understanding hydration, gelation, and viscoelasticity of gluten networks in wheat-based food systems.
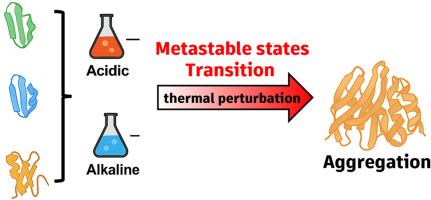
通过全原子分子动力学模拟揭示HMW-GSs的ph驱动亚稳跃迁和热聚集
高分子量谷蛋白亚基(HMW-GSs)是小麦面团粘弹性的关键贡献者,其构象适应性在面筋网络的形成中起着至关重要的作用。在这项研究中,我们采用了两阶段分子动力学模拟方法来研究酸性(pH 2)和碱性(pH 12)条件如何调节三种x型HMW-GSs-Ax1、Bx7和dx2的亚稳态,以及这些构象如何响应热聚集。结果显示了明显的亚基特异性构象可塑性差异。在极端pH条件下,与Dx2相比,Ax1和Bx7表现出更大的RMSD波动和更宽的旋转半径分布,表明结构灵活性增强。氢键寿命分析表明,Bx7在亚稳态之间保持了更长的相互作用,这意味着更大的内部稳定性。在400 K的热加速下,Ax1迅速向易于聚集的构象发展,而Dx2保留了部分结构完整性,反映了其优越的热弹性。基于协方差的互相关分析进一步显示,Ax1和Bx7中存在广泛的域解耦,而Dx2的动态则更为协调。通过将亚稳态集成与聚集行为联系起来,本研究提供了谷蛋白适应性的机制见解。这些分子的见解也可以作为理解水合作用、凝胶作用和面筋网络在小麦为基础的食物系统中的粘弹性的参考。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Hydrocolloids
工程技术-食品科技
CiteScore
19.90
自引率
14.00%
发文量
871
审稿时长
37 days
期刊介绍:
Food Hydrocolloids publishes original and innovative research focused on the characterization, functional properties, and applications of hydrocolloid materials used in food products. These hydrocolloids, defined as polysaccharides and proteins of commercial importance, are added to control aspects such as texture, stability, rheology, and sensory properties. The research's primary emphasis should be on the hydrocolloids themselves, with thorough descriptions of their source, nature, and physicochemical characteristics. Manuscripts are expected to clearly outline specific aims and objectives, include a fundamental discussion of research findings at the molecular level, and address the significance of the results. Studies on hydrocolloids in complex formulations should concentrate on their overall properties and mechanisms of action, while simple formulation development studies may not be considered for publication.
The main areas of interest are:
-Chemical and physicochemical characterisation
Thermal properties including glass transitions and conformational changes-
Rheological properties including viscosity, viscoelastic properties and gelation behaviour-
The influence on organoleptic properties-
Interfacial properties including stabilisation of dispersions, emulsions and foams-
Film forming properties with application to edible films and active packaging-
Encapsulation and controlled release of active compounds-
The influence on health including their role as dietary fibre-
Manipulation of hydrocolloid structure and functionality through chemical, biochemical and physical processes-
New hydrocolloids and hydrocolloid sources of commercial potential.
The Journal also publishes Review articles that provide an overview of the latest developments in topics of specific interest to researchers in this field of activity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


