Selective Ammonia Electrooxidation on TiB₄ and VB₄ quantum dots: Theoretical insights into catalytic activity and OER suppression
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
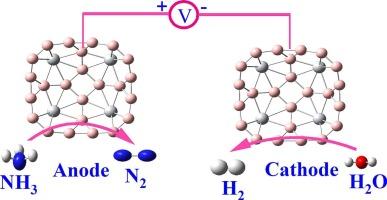
Ammonia (NH₃) has emerged as a promising carbon-free hydrogen carrier for sustainable energy applications. However, the development of cost-effective and efficient electrocatalysts for the ammonia oxidation reaction (AOR) remains a significant challenge. In this work, we employ density functional theory (DFT) calculations to investigate the structural stability, electronic properties, and catalytic performance of TiB₄ and VB₄ quantum dots (QDs) toward AOR. Structural optimizations, Mulliken charge analysis, and binding energy assessments confirm the thermodynamic stability of both QDs, with TiB₄ exhibiting slightly higher stability. Adsorption energy analysis reveals a strong affinity of both QDs for NH₃, with preferential binding at the transition metal centers. Reaction mechanisms were explored through both the Oswin–Salomon (O![]() S) and Gerischer–Mauerer (G–M) pathways, and corresponding free energy diagrams highlight TiB₄–S1 as the most promising active site. It exhibits remarkably low overpotentials of 0.084 V and 0.655 V for the O
S) and Gerischer–Mauerer (G–M) pathways, and corresponding free energy diagrams highlight TiB₄–S1 as the most promising active site. It exhibits remarkably low overpotentials of 0.084 V and 0.655 V for the O![]() S and G–M mechanisms, respectively—outperforming benchmark noble-metal catalysts such as Pt(100) and Fe/Pt(100). In contrast, VB₄ QDs suffer from high energy barriers and less favourable reaction energetics. Furthermore, comparative analysis with the competing oxygen evolution reaction (OER) confirms that TiB₄ QDs exhibit superior selectivity for AOR under anodic conditions. These findings position TiB₄ QDs as highly efficient, non-noble metal-based electrocatalysts for AOR, offering great potential for integration into next-generation fuel cells and green hydrogen technologies.
S and G–M mechanisms, respectively—outperforming benchmark noble-metal catalysts such as Pt(100) and Fe/Pt(100). In contrast, VB₄ QDs suffer from high energy barriers and less favourable reaction energetics. Furthermore, comparative analysis with the competing oxygen evolution reaction (OER) confirms that TiB₄ QDs exhibit superior selectivity for AOR under anodic conditions. These findings position TiB₄ QDs as highly efficient, non-noble metal-based electrocatalysts for AOR, offering great potential for integration into next-generation fuel cells and green hydrogen technologies.
TiB₄和VB₄量子点上选择性氨电氧化:催化活性和OER抑制的理论见解
氨(NH₃)已经成为一种有前途的无碳氢载体,用于可持续能源的应用。然而,开发经济高效的氨氧化反应电催化剂仍然是一个重大挑战。在这项工作中,我们采用密度泛函理论(DFT)计算研究了TiB₄和VB₄量子点(QDs)对AOR的结构稳定性、电子性质和催化性能。结构优化、Mulliken电荷分析和结合能评估证实了这两个量子点的热力学稳定性,其中TiB₄的稳定性略高。吸附能分析表明,这两个量子点对NH₃有很强的亲和力,在过渡金属中心有优先结合。通过Oswin-Salomon (OS)和Gerischer-Mauerer (G-M)两种途径探索了反应机理,相应的自由能图显示TiB₄-S1是最有希望的活性位点。OS和G-M机制的过电位分别为0.084 V和0.655 V,优于基准贵金属催化剂Pt(100)和Fe/Pt(100)。相反,VB₄量子点具有高能量垒和较差的反应能。此外,通过与OER(竞争性析氧反应)的对比分析,证实了TiB₄QDs在阳极条件下对AOR具有更好的选择性。这些发现将TiB₄QDs定位为高效的非贵金属基AOR电催化剂,为下一代燃料电池和绿色氢技术的集成提供了巨大的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


