Synthesis and characterization of a functional benzoylecgonine – bovine serum albumin conjugate for quantification of a humanized monoclonal anti-cocaine antibody
IF 2.2
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
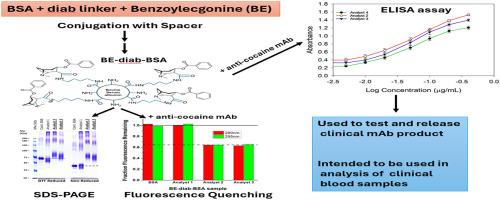
We have developed a humanized monoclonal anti-cocaine antibody (h2E2), as a potential solution for cocaine use disorder. A key milestone was the development of an assay to quantify this monoclonal antibody (mAb) in animal and human blood. Thus, we synthesized a novel benzoylecgonine-1,4-diaminobutane-BSA (BE-diab BSA) conjugate as an antigen for quantifying h2E2 using ELISA. We report here the method of synthesis of this conjugate, BE-diab BSA, and assessment of its binding to the h2E2 mAb using an ELISA and a fluorescence quenching assay. Compared with four commercial BE-BSA conjugates, BE-diab BSA demonstrated markedly stronger mAb binding in ELISA—three of the commercial conjugates showed less than 10 % relative binding. Fluorescence quenching assays confirmed this binding superiority, with the commercial conjugates showing minimal mAb interaction, while BE-diab BSA induced robust intrinsic fluorescence quenching. SDS-PAGE analyses identified structural differences consistent with binding results between our functional conjugate and the commercial preparations. This functional and reproducible in-house conjugation has been integrated into a GLP-validated ELISA, which is now in use for pharmacokinetic analyses and for qualifying antibody release lots for clinical deployment.
用于人源化抗可卡因单克隆抗体定量分析的功能性苯甲酰ecgonine -牛血清白蛋白偶联物的合成与鉴定
我们已经开发了一种人源化单克隆抗可卡因抗体(h2E2),作为可卡因使用障碍的潜在解决方案。一个关键的里程碑是开发了一种定量动物和人血液中这种单克隆抗体(mAb)的测定方法。因此,我们合成了一种新的苯甲酰卵磷脂-1,4-二氨基丁烷-BSA (BE-diab BSA)偶联物,作为ELISA定量h2E2的抗原。我们在这里报道了这种缀合物BE-diab BSA的合成方法,并使用ELISA和荧光猝灭试验评估其与h2E2单抗的结合。与四种商业BE-BSA偶联物相比,BE-diab BSA在elisa中表现出明显更强的单抗结合,其中三种商业偶联物的相对结合率低于10%。荧光猝灭实验证实了这种结合优势,商业偶联物显示出最小的单抗相互作用,而BE-diab BSA诱导了强大的内在荧光猝灭。SDS-PAGE分析发现我们的功能偶联物和商业制剂之间的结构差异与结合结果一致。这种功能和可重复的内部偶联已集成到glp验证的ELISA中,目前用于药代动力学分析和确定临床部署的抗体释放批次。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemistry and Biophysics Reports
Biochemistry, Genetics and Molecular Biology-Biophysics
CiteScore
4.60
自引率
0.00%
发文量
191
审稿时长
59 days
期刊介绍:
Open access, online only, peer-reviewed international journal in the Life Sciences, established in 2014 Biochemistry and Biophysics Reports (BB Reports) publishes original research in all aspects of Biochemistry, Biophysics and related areas like Molecular and Cell Biology. BB Reports welcomes solid though more preliminary, descriptive and small scale results if they have the potential to stimulate and/or contribute to future research, leading to new insights or hypothesis. Primary criteria for acceptance is that the work is original, scientifically and technically sound and provides valuable knowledge to life sciences research. We strongly believe all results deserve to be published and documented for the advancement of science. BB Reports specifically appreciates receiving reports on: Negative results, Replication studies, Reanalysis of previous datasets.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


