Mechanisms of the loading of ibuprofen into UiO-66s in various solvents: A combined experimental and MC simulation study
IF 4.7
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Metal-organic frameworks (MOFs), which exhibit high performances as drug carriers, attract increasing attention in drug delivery systems. The liquid-phase loading of drugs into MOFs is critical in determining their performances as drug carriers. Here, this study is the first to evaluate experimentally the influences of solvent polarity and the functional groups on drug-loading capacity and elucidate quantitatively mechanisms focusing on the MOF-drug-solvent interactions by grand canonical Monte Carlo (GCMC) and canonical MC (CMC) simulations. The drug-loading capacities of Universitetet i Oslo-66-X (X = NH2, H, Br, NO2) were investigated via experiments and molecular simulations. Experimentally, the drug-loading amount decreased with an increase in the solvent dipole moment and the shift of the functional groups from electron-donating to -withdrawing. These trends were attributed to variations in the MOF-drug and drug-solvent interactions. By combining the GCMC and CMC simulations, we investigated on the relationships between the energy components (|ΔEMOF–solvent|, |E2,MOF–drug|, and |E2,drug–solvent|) and experimental drug-loading capacity. The balance of the intermolecular interactions between the MOF, drug, and solvent, particularly MOF-solvent interaction, was crucial in determining the drug-loading behavior. In ethanol, a higher MOF-drug interaction energy and lower drug-solvent interaction energy corresponded to a higher experimental drug loading. Overall, solvent dipole moment, electronic natures of the functional groups of the MOF, and the balance of MOF-drug-solvent interactions determined the drug-loading performance. These findings provide insights for use in the rational design of MOF-based drug carriers and contribute to a deeper understanding of adsorption behavior in the liquid phase.
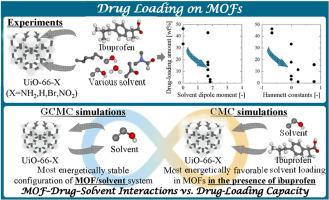
不同溶剂中布洛芬在UiO-66s中的负载机制:实验与MC模拟相结合的研究
金属有机骨架(MOFs)作为一种高性能的药物载体,在给药系统中受到越来越多的关注。液相载药是决定mof作为药物载体性能的关键。本研究首次通过实验评估了溶剂极性和官能团对载药量的影响,并通过大正则蒙特卡罗(GCMC)和正则MC (CMC)模拟,定量阐明了mof -药物-溶剂相互作用的机制。通过实验和分子模拟研究了奥斯陆大学66-X (X = NH2, H, Br, NO2)的载药能力。实验结果表明,随着溶剂偶极矩的增大和官能团由供电子向吸电子的转移,载药量减小。这些趋势归因于mof -药物和药物-溶剂相互作用的变化。结合GCMC和CMC模拟,我们研究了能量组分(| ΔEMOF-solvent |, |E2, MOF-drug |和|E2,药物-溶剂|)与实验载药量的关系。MOF、药物和溶剂之间的分子间相互作用的平衡,特别是MOF-溶剂相互作用,是决定载药行为的关键。在乙醇中,较高的mof -药物相互作用能和较低的药物-溶剂相互作用能对应着较高的实验药物负荷。总的来说,溶剂偶极矩、MOF官能团的电子性质以及MOF-溶剂相互作用的平衡决定了MOF-溶剂的载药性能。这些发现为合理设计基于mof的药物载体提供了见解,并有助于更深入地了解液相中的吸附行为。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


