From sublimation to tautomerization and aggregation: Infrared spectroscopic insights into ammonium thiocyanate dissociation at low temperatures
IF 2.4
3区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The dissociation of ammonium thiocyanate (NH4SCN) during the sublimation of NH4SCN to form isothiocyanic acid (HNCS; a tautomer of HSCN) and ammonia (NH3) was evidenced by matrix isolation infrared spectroscopy. HNCS was observed to behave differently within Ar and N2, on annealing the matrices. Formation of homodimers of HNCS is notably more rapid in N2 than in Ar. Interestingly, the infrared spectra of solid film of NH4SCN at 10 K obtained by refreezing sublimed NH4SCN did not show any characteristic dissociation. Experimental results were corroborated with the computations performed at B3LYP-GD3/aug-cc-pVDZ level.
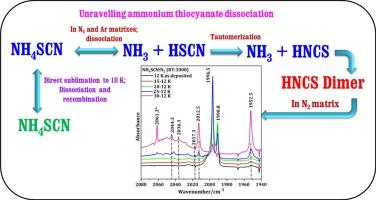
从升华到互变异构和聚集:低温下硫氰酸铵解离的红外光谱研究
基质分离红外光谱证实了硫氰酸铵(NH4SCN)在升华过程中解离生成异硫氰酸(HNCS, HSCN的互变异构体)和氨(NH3)。退火后,观察到HNCS在Ar和N2中表现出不同的行为。nhcs的同型二聚体在N2中的形成速度明显快于Ar。有趣的是,在10 K时重新冷冻升华的NH4SCN固体膜的红外光谱没有显示出任何特征的解离。实验结果与B3LYP-GD3/aug-cc-pVDZ水平的计算结果一致。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
4.60
自引率
4.30%
发文量
278
审稿时长
39 days
期刊介绍:
Chemical Physics publishes experimental and theoretical papers on all aspects of chemical physics. In this journal, experiments are related to theory, and in turn theoretical papers are related to present or future experiments. Subjects covered include: spectroscopy and molecular structure, interacting systems, relaxation phenomena, biological systems, materials, fundamental problems in molecular reactivity, molecular quantum theory and statistical mechanics. Computational chemistry studies of routine character are not appropriate for this journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


