Neuronal regulation of myenteric interstitial cells of Cajal (ICC-MY) in the proximal colon
IF 4
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
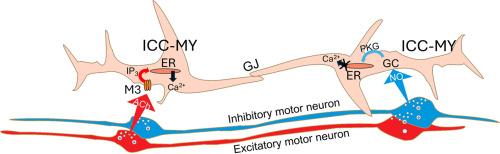
Interstitial cells of Cajal (ICC) generate contractile patterns of colonic motility. We investigated innervation of ICC within the plane of the myenteric plexus (ICC-MY) in proximal colon using mice expressing GCaMP6f in ICC. ICC-MY generated localized Ca2+ transients that couple to activation of ANO1 channels, a Ca2+-activated Cl- conductance. ICC are electrically coupled to SMCs, so activation or suppression of currents in ICC affects excitability of SMCs. ICC-MY displayed tonic inhibition, as the neurotoxin, TTX, increased the frequency of Ca2+ transients. Tonic inhibition was mimicked by a nitric oxide donor, NONOate, and by a guanylate cyclase agonist (Bay 58–2667). In contrast ODQ mimicked effects of TTX, increasing Ca2+ transients. Carbachol (CCh) increased Ca2+ transients in ICC-MY, and these effects were mediated by M3 muscarinic receptors. Neostigmine, also increased Ca2+ transients, suggesting there is tonic activation of enteric excitatory neurons in colonic muscles. Substance P and antagonists of NK1 and NK2 receptors had no effect on Ca2+ transients in ICC-MY. Electrical field stimulation (EFS), under conditions that emphasized excitatory neural responses, enhanced Ca2+ transients, and these effects were blocked by atropine or an M3 receptor antagonist (DAU 5884). EFS in the presence of atropine caused inhibition of Ca2+ via release of NO. Cessation of nitrergic stimulation resulted in a substantial increase in Ca2+ transients, known as post-stimulus excitation. In summary, ICC-MY, important for the generation of propulsive contractions in the colon, are innervated by excitatory (cholinergic) and inhibitory (nitrergic) motor neurons, and these inputs regulate the excitability of these cells.
结肠近端Cajal肌肠间质细胞(ICC-MY)的神经元调控
Cajal间质细胞(ICC)产生结肠运动的收缩模式。我们利用在ICC中表达GCaMP6f的小鼠,研究了ICC在近端结肠肌肠丛平面(ICC- my)内的神经支配。ICC-MY产生局部Ca2+瞬态,偶联激活ANO1通道,Ca2+激活的Cl-电导。ICC与SMCs是电耦合的,因此ICC中电流的激活或抑制会影响SMCs的兴奋性。ICC-MY表现出强直性抑制,因为神经毒素TTX增加了Ca2+瞬态的频率。滋补抑制由一氧化氮供体NONOate和鸟苷酸环化酶激动剂模拟(Bay 58-2667)。相反,ODQ模拟了TTX的作用,增加了Ca2+瞬态。碳碱(CCh)增加了ICC-MY中的Ca2+瞬态,这些作用是由M3毒蕈碱受体介导的。新斯的明也增加Ca2+瞬态,提示结肠肌肉的肠兴奋性神经元存在强直性激活。P物质和NK1和NK2受体拮抗剂对ICC-MY中的Ca2+瞬态没有影响。电场刺激(EFS),在强调兴奋性神经反应的条件下,增强Ca2+瞬态,这些作用被阿托品或M3受体拮抗剂(DAU 5884)阻断。在阿托品存在下的EFS通过释放NO引起Ca2+的抑制。氮能刺激的停止导致Ca2+瞬态的大量增加,称为刺激后兴奋。总之,ICC-MY对结肠推进性收缩的产生很重要,它受兴奋性(胆碱能)和抑制性(氮能)运动神经元的支配,这些输入调节这些细胞的兴奋性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Cell calcium
生物-细胞生物学
CiteScore
8.70
自引率
5.00%
发文量
115
审稿时长
35 days
期刊介绍:
Cell Calcium covers the field of calcium metabolism and signalling in living systems, from aspects including inorganic chemistry, physiology, molecular biology and pathology. Topic themes include:
Roles of calcium in regulating cellular events such as apoptosis, necrosis and organelle remodelling
Influence of calcium regulation in affecting health and disease outcomes
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


