Bio-templated green synthesis of ZnO nanostructures using herbal seed Mucilages: A sustainable route for dye adsorption
IF 4.3
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Conventional methods for removing toxic dyes from aqueous media, often impose high costs or result in the release of secondary pollutions. So as an alternative solution, utilization and assessment of sustainable adsorbents obtained from green chemistry principles are interesting and progressive. This study investigates the synthesis, characterization, and adsorption performance of herbal-mediated zinc oxide (ZnO) nanoparticles to remove Congo red (CR) dye from aqueous solutions. The novelty of this study lies in the bio-templated green hydrothermal synthesis of ZnO nanoparticles using mucilages extracted from four herbal seeds, such as Plantago ovata, Alyssum homalocarpum, Plantago major, and Cydonia oblonga as biogenic directing agents. This approach marks the first report of such a morphologically controlled synthesis using the plant-based precursors, yielding a nanopowder with high crystallinity and mesoporous architecture (average pore size of 24.49 nm and surface area of 10.36 m²/g). Adsorption studies revealed a maximum capacity of 120.48 mg/g, following pseudo-second-order kinetics (R² = 0.9929) and the Langmuir isotherm model (R²=0.997). Thermodynamic analysis confirmed the exothermic nature of the process, with spontaneous and entropy-driven characteristics. The adsorbent exhibited pH-dependent performance, with optimal removal efficiency (98 %) at acidic conditions, attributed to electrostatic interactions between protonated ZnO surfaces and anionic CR species. Regeneration studies showed a 25 % capacity loss over five cycles, linked to mesopore occlusion and surface hydroxyl depletion. This work demonstrates the potential of plant-mediated ZnO as a sustainable adsorbent for textile wastewater treatment while providing mechanistic insights into its structure-performance relationship.
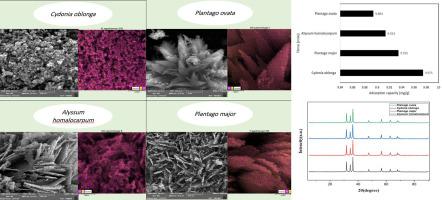
植物种子粘液生物模板绿色合成氧化锌纳米结构:染料吸附的可持续途径
从水介质中去除有毒染料的传统方法往往成本高或导致二次污染的释放。因此,作为一种替代方案,利用和评估从绿色化学原理获得的可持续吸附剂是有趣的和进步的。本文研究了草药介导氧化锌纳米颗粒的合成、表征和对刚果红染料的吸附性能。本研究的新颖之处在于以四种草本植物种子(车前子、鸢尾、大车前子和Cydonia oblonga)为生物导向剂,采用生物模板绿色水热法合成ZnO纳米颗粒。该方法标志着使用基于植物的前体进行这种形态控制合成的首次报道,产生了具有高结晶度和介孔结构的纳米粉末(平均孔径为24.49 nm,表面积为10.36 m²/g)。根据拟二级动力学方程(R²= 0.9929)和Langmuir等温模型(R²=0.997),吸附量最大可达120.48 mg/g。热力学分析证实了该过程的放热性质,具有自发和熵驱动的特征。该吸附剂表现出ph依赖性,在酸性条件下具有最佳的去除效率(98%),这归因于质子化ZnO表面和阴离子CR之间的静电相互作用。再生研究表明,在五个循环中,25%的容量损失与介孔堵塞和表面羟基耗尽有关。这项工作证明了植物介导的氧化锌作为纺织废水处理的可持续吸附剂的潜力,同时提供了其结构-性能关系的机理见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Physics Impact
Materials Science-Materials Science (miscellaneous)
CiteScore
2.60
自引率
0.00%
发文量
65
审稿时长
46 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


