Ticagrelor-loaded lipid nanocapsules as a promising nebulizable system for lung cancer: in-vitro characterization, 131I radiolabeling and in-vivo biodistribution
IF 4.9
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2025-09-21
DOI:10.1016/j.jddst.2025.107560
引用次数: 0
Abstract
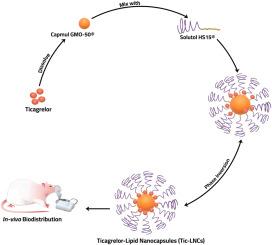
This study investigates the encapsulation of Ticagrelor (Tic) within lipid nanocapsules (LNCs) as a potential nebulizable drug delivery system for the treatment of lung cancer. The prepared LNCs were evaluated through in-vitro characterization, including dynamic light scattering (DLS) and surface morphology. Additionally, an in-vivo biodistribution study was conducted after radiolabeling the selected Tic-LNCs with iodine-131 (131I) to assess their distribution and deposition within various body organs. Results indicated that the Tic-LNCs had a particle size ranging from 137.5 ± 0.4 nm to 340.15 ± 17.3 nm. The in-vitro cytotoxicity using the MTT assay showed that Tic-LNCs exerted a substantial inhibitory effect against A549 lung cancer cells, with IC50 values of Tic and Tic-LNCs being 21.72 ± 1.22 μg/mL and 3.42 ± 0.103 μg/mL, respectively. The in-vivo biodistribution study revealed that the 131I-Tic-LNCs demonstrated significantly better lung uptake compared to the parent drug solution. The lung-to-blood ratio for the Tic-LNCs reached a maximum of 2.67 within 4 h after administration. In conclusion, the nebulizable Tic-LNCs nanosystem holds promise as a targeted pulmonary drug delivery system for the treatment of lung cancer.
替格瑞洛负载脂质纳米胶囊作为一种有前途的肺癌雾化系统:体外表征,131I放射性标记和体内生物分布
本研究探讨了替格瑞洛(Tic)在脂质纳米胶囊(LNCs)内的包封,作为治疗肺癌的潜在雾化药物输送系统。通过动态光散射(DLS)和表面形貌等体外表征对制备的LNCs进行评价。此外,在用碘-131 (131I)对选定的Tic-LNCs进行放射性标记后,进行了体内生物分布研究,以评估它们在人体各器官中的分布和沉积。结果表明,Tic-LNCs的粒径范围为137.5±0.4 nm ~ 340.15±17.3 nm。MTT法体外细胞毒性实验表明,Tic- lncs对A549肺癌细胞具有明显的抑制作用,其IC50值分别为21.72±1.22 μg/mL和3.42±0.103 μg/mL。体内生物分布研究显示,与母体药物溶液相比,131I-Tic-LNCs表现出明显更好的肺摄取。Tic-LNCs的肺血比在给药后4 h内达到最大值2.67。总之,可雾化的Tic-LNCs纳米系统有望成为治疗肺癌的靶向肺药物输送系统。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


