High-purity cochleates engineered with DOTAP: Unlocking new potential for drug delivery
IF 4.9
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2025-09-22
DOI:10.1016/j.jddst.2025.107557
引用次数: 0
Abstract
Cochleates are multilamellar lipid–calcium precipitates with potential for oral delivery of poorly soluble or irritant drugs, but their heterogeneity has limited translation. Here, we report the engineering of highly pure, structurally uniform cochleates using a cationic lipid (DOTAP) in combination with anionic phospholipids (DOPS, DMPS). CAP (capsaicin) was selected as a model drug due to its poor solubility, instability, and gastrointestinal side effects. DOTAP-containing cochleates (H) displayed well-defined rod-like morphology, reduced aggregation, and enhanced colloidal stability compared to conventional formulations (A–G). Physicochemical analysis (FE-SEM, SAXS, DSC, FTIR) confirmed strong CAP–lipid interactions, reduced crystallinity, and improved bilayer ordering. Encapsulation efficiency reached 93.3 % with loading of 196 mg/g, markedly higher than typical nanocarriers. In-vitro release showed suppressed burst effect and sustained diffusion-controlled release over 94 h, with cochleates H exhibiting the slowest release kinetics. Stability studies demonstrated excellent drug retention at physiological pH and moderate temperatures. Together, these findings establish DOTAP-engineered cochleates as a robust platform for oral delivery, offering high purity, reproducibility, and translational promise for hydrophobic or irritant drugs.
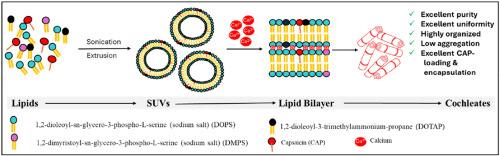
用DOTAP设计的高纯度耳蜗:释放药物传递的新潜力
耳蜗酸盐是多层脂钙沉淀物,具有口服递送难溶性或刺激性药物的潜力,但其异质性限制了翻译。在这里,我们报道了使用阳离子脂质(DOTAP)与阴离子磷脂(DOPS, DMPS)结合的高纯度,结构均匀的耳蜗酸酯的工程。CAP(辣椒素)由于其溶解度差、不稳定性和胃肠道副作用而被选为模型药物。与常规配方(A-G)相比,含有dotap的耳蜗(H)表现出明确的棒状形态,减少了聚集,增强了胶体稳定性。理化分析(FE-SEM, SAXS, DSC, FTIR)证实了cap -脂质的强相互作用,降低了结晶度,改善了双分子层的有序性。当载药量为196 mg/g时,包封率达到93.3%,明显高于典型的纳米载体。体外释放表现出抑制爆发效应和持续扩散控制释放超过94 h,其中耳蜗酸酯h的释放动力学最慢。稳定性研究表明,在生理pH值和中等温度下,药物保持良好。总之,这些发现确立了dotap工程耳蜗作为口服给药的强大平台,具有高纯度、可重复性和对疏水或刺激性药物的转化前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


