Formulation matters: Assessment of the correlation between mucoadhesiveness and type of chitosan formulation for vaginal application
IF 4.9
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2025-09-22
DOI:10.1016/j.jddst.2025.107564
引用次数: 0
Abstract
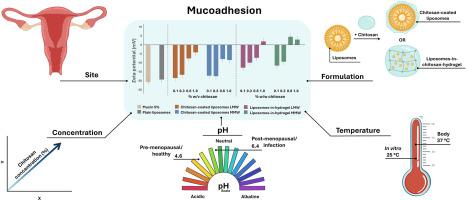
Localized vaginal drug delivery can offer safer and more effective treatment options for various conditions, also for pregnant women. However, inadequate retention of formulation in the vaginal cavity hinders efficient treatment. Mucoadhesive drug delivery systems using mucoadhesive polymers may be a promising solution. In this work, the biopolymer chitosan was chosen for its well documented mucoadhesive properties, favorable biopharmaceutical profile, and low toxicity. However, its mucoadhesive abilities may depend on how chitosan is formulated. This study therefore focused on two distinct chitosan formulations, chitosan-coated liposomes and liposomes-in-chitosan hydrogel, to compare mucoadhesiveness across formulations. Additionally, two chitosan molecular weights (LMW and MMW) and four concentrations (0.1 %, 0.3 %, 0.6 % and 1.0 %) were examined, whilst considering site-specific factors like pH, biological fluids, and temperature. The formulations were characterized, and their mucoadhesive behavior evaluated by monitoring changes in viscosity and zeta potential upon mixing with mucin. Liposomes averaged 135 nm in size with a zeta potential of −2.78 mV, where the charge increased with chitosan coating or incorporation into chitosan hydrogel. Both chitosan-coated liposomes and liposomes-in-hydrogel exhibited rheological properties suitable for vaginal application. Mucoadhesion indeed varied based on the formulation, chitosan concentration, molecular weight, pH, and temperature. Notably, liposomes-in-hydrogel formulations demonstrated superior mucoadhesive potential. These findings emphasize the importance of formulation design and environmental factors in optimizing mucoadhesion.
制剂问题:评估黏附性与阴道应用的壳聚糖制剂类型之间的相关性
局部阴道给药可以为各种疾病提供更安全、更有效的治疗选择,对孕妇也是如此。然而,不适当的保留配方在阴道腔阻碍有效的治疗。使用黏附聚合物的黏附给药系统可能是一种很有前途的解决方案。在这项工作中,选择了生物聚合物壳聚糖,因为它具有良好的粘接性能,良好的生物制药特性和低毒性。然而,它的粘接能力可能取决于壳聚糖的配方。因此,本研究的重点是两种不同的壳聚糖配方,壳聚糖包被脂质体和壳聚糖水凝胶中的脂质体,以比较不同配方的黏附性。此外,在考虑pH、生物流体和温度等特定位点因素的情况下,研究了两种壳聚糖分子量(LMW和MMW)和四种浓度(0.1%、0.3%、0.6%和1.0%)。对配方进行了表征,并通过监测与粘蛋白混合后粘度和zeta电位的变化来评估其粘接行为。脂质体平均尺寸为135 nm, zeta电位为- 2.78 mV,其中电荷随着壳聚糖涂层或壳聚糖水凝胶的加入而增加。壳聚糖包被脂质体和水凝胶脂质体均表现出适合阴道应用的流变特性。黏附确实根据配方、壳聚糖浓度、分子量、pH值和温度而变化。值得注意的是,水凝胶中的脂质体制剂显示出优越的粘接潜力。这些发现强调了配方设计和环境因素对优化黏附的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


