Comparison of Hydroxylamine Hydrochloride and Hydrogen Peroxide in Activated Fe(VI) for Fracturing Flowback Fluid Treatment: Efficiency, Mechanism, and Toxicity Analysis
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Ferrate(VI) oxidation is an effective approach for organic wastewater treatment. However, its application in fracturing flowback fluid with high salinity and complex contaminants requires further investigation. In this study, two distinct Fe(VI) activation strategies, hydroxylamine hydrochloride and hydrogen peroxide, were applied to enhance contaminants oxidation in fracturing flowback fluid. The results showed HA facilitated Fe(IV)/Fe(V) generation by electron transfer, whereas exogenous H2O2 enhanced ·OH generation. Both Fe(VI) activated systems improved pollutant degradation compared with Fe(VI) alone. Under optimal conditions, Fe(VI)-H2O2 system dominated by ·OH exhibited better organic removal (TOC removal 46.0%, UV254 removal 66.6%, and the BOD5/COD value (the B/C) 0.436) than Fe(VI)-HA system dominated by Fe(IV)/Fe(V) (TOC removal 33.7%, UV254 removal 61.5%, and the B/C 0.332). The organic species number in the Fe(VI)-H2O2 system was reduced by nearly half than Fe(VI)-HA, and the system can completely remove the alcohols, acids, and ether substances. In addition, toxicity tests showed that in high salinity wastewater (Cl- and salinity concentration of 5498.9 and 10332.0 mg/L), both HA and H2O2 activated Fe(VI) strategies can decrease the acute toxicity and phytotoxicity. Overall, both HA and H2O2-activated Fe(VI) oxidation are feasible for treating fracturing flowback fluid, with H2O2-activated Fe(VI) oxidation exhibiting higher efficacy.
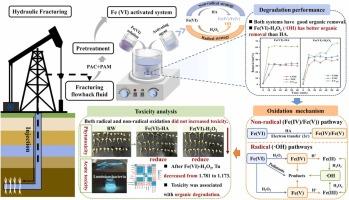
盐酸羟胺与过氧化氢在活性铁(VI)中处理压裂返排液的效果、机理及毒性分析
高铁酸盐(VI)氧化法是处理有机废水的有效方法。但其在高矿化度、复杂污染物的压裂返排液中的应用还有待进一步研究。在这项研究中,采用了两种不同的Fe(VI)活化策略——盐酸羟胺和过氧化氢——来增强压裂返排液中的污染物氧化。结果表明,HA通过电子传递促进了Fe(IV)/Fe(V)的生成,而外源H2O2促进了·OH的生成。与单独使用Fe(VI)相比,两种Fe(VI)活化体系都能改善污染物的降解。在最佳条件下,以·OH为主的Fe(VI)-H2O2体系的有机物去除率(TOC去除率46.0%,UV254去除率66.6%,BOD5/COD值(B/C) 0.436)优于以Fe(IV)/Fe(V)为主的Fe(VI)-HA体系(TOC去除率33.7%,UV254去除率61.5%,B/C 0.332)。Fe(VI)-H2O2体系中的有机物种类数量比Fe(VI)-HA减少了近一半,并且该体系可以完全去除醇类、酸类和醚类物质。此外,毒性试验表明,在高盐度废水(Cl-和盐度浓度分别为5498.9和10332.0 mg/L)中,HA和H2O2激活Fe(VI)策略均可降低其急性毒性和植物毒性。综上所述,HA和h2o2活化Fe(VI)氧化处理压裂返排液都是可行的,其中h2o2活化Fe(VI)氧化效果更好。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


