Synergistic Effects of Micro/Nanoplastics and Cu(II) on Horizontal Transfer of Antibiotic Resistance Genes: New insight targeting on Cell Surface Properties
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Microplastics (MPs) and nanoplastics (NPs) facilitate antibiotic resistance genes (ARGs) transfer through horizontal gene transfer (HGT). However, the combined effects of M-NPs and heavy metals on HGT remain poorly understood, and the effects of cell surface properties is neglected. In this study, an antibiotic co-existence heavy metal Cu was used to study its synergetic effect with M-NPs on HGT, with a specific focus on bacterial surface characteristics and physiological responses. Results reveal that NPs amplified Cu(II)'s effect on conjugative transfer of ARGs, while MPs showed mitigation effect. NPs+Cu(II) co-exposure yielded the highest conjugative transfer frequency (4.4-fold) and a 35-fold surge in transformation frequency compared to the control. These disparities stem from bacterial physiological responses, including 4–7-fold elevated reactive oxygen species (ROS), 3–4-fold increased membrane permeability, 1.5–1.8-fold enhanced ATP synthesis, altered drug-resistant efflux and metabolic pathways; Furthermore, cell surface property modulation—Cu(II) stimulated 1.2-fold lipopolysaccharide (LPS) production and M-NPs regulated outer membrane vesicles (OMVs) concentration/sizes, with extracellular polymeric substances (EPS) optimizing interbacterial aggregation for gene transfer. In addition, MPs+Cu(II) induced 49% viable but non-culturable (VBNC) bacteria and high-dose M-NPs caused excessive bacterial injury/death, reducing gene transfer (VBNC ratio indicating stress severity). These findings highlight co-exposure impacts and offer novel insights into the environmental risks posed by M-NPs and ARGs.
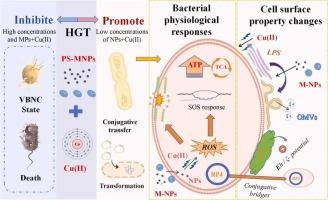
微/纳米塑料和Cu(II)在抗生素抗性基因水平转移中的协同作用:针对细胞表面特性的新见解
微塑料(MPs)和纳米塑料(NPs)通过水平基因转移(HGT)促进抗生素抗性基因(ARGs)的转移。然而,M-NPs和重金属对HGT的综合影响仍然知之甚少,并且忽略了细胞表面特性的影响。本研究采用抗生素共存重金属Cu,研究其与M-NPs对HGT的协同作用,重点关注细菌表面特征和生理反应。结果表明,NPs增强了Cu(II)对ARGs共轭转移的影响,而MPs则表现出抑制作用。与对照组相比,NPs+Cu(II)共暴露产生了最高的共轭转移频率(4.4倍)和35倍的转换频率激增。这些差异源于细菌的生理反应,包括活性氧(ROS)增加4- 7倍,膜通透性增加3 - 4倍,ATP合成增加1.5 - 1.8倍,耐药外排和代谢途径改变;此外,细胞表面特性调节- cu (II)刺激1.2倍的脂多糖(LPS)产生,M-NPs调节外膜囊泡(OMVs)的浓度/大小,细胞外聚合物质(EPS)优化细菌间聚集以进行基因转移。此外,MPs+Cu(II)诱导了49%的活菌(VBNC),高剂量M-NPs导致了过多的细菌损伤/死亡,减少了基因转移(VBNC比率表明应激严重程度)。这些发现突出了共同暴露的影响,并为M-NPs和ARGs带来的环境风险提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


