Discovery and validation of LAGi-DEL: A nanomolar LAG-3 inhibitor that reverses immune suppression in tumor-immune co-culture models
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Lymphocyte activation gene-3 (LAG-3) is an immune checkpoint receptor that drives T cell exhaustion and tumor immune evasion. While antibody-based LAG-3 inhibitors have entered clinical practice, their poor tumor penetration, high manufacturing costs, and prolonged half-lives limit dosing flexibility and can lead to extended immune-related toxicities. Potent small molecule LAG-3 inhibitors remain elusive, leaving a major gap in the immuno-oncology landscape. Here, we report the discovery of LAGi-DEL, the first nanomolar small molecule LAG-3 inhibitor, identified through screening a 4.2-billion compound DNA-encoded chemical library (DEL). LAGi-DEL binds directly to LAG-3 with a Kd of 97 nM (SPR) and 271 nM (MST) and disrupts the LAG-3/MHC-II interaction with an EC50 of 138 nM, the highest potency reported for a small molecule against LAG-3. Molecular docking and dynamics simulations reveal that LAGi-DEL induces stabilizing conformational changes within the LAG-3 binding pocket. Functionally, LAGi-DEL restores T cell activation, elevates IFN-γ secretion, and promotes immune-mediated killing in acute myeloid leukemia and lung cancer co-culture models, matching the efficacy of the FDA-approved antibody relatlimab. Pharmacokinetic profiling shows favorable drug-like properties, including high solubility, acceptable permeability, robust metabolic and plasma stability, low cytotoxicity toward normal cells, and negligible hERG and CYP liabilities. These attributes, combined with broad functional activity and unprecedented potency, position LAGi-DEL as a promising scaffold for developing orally available LAG-3 inhibitors and as a blueprint for next-generation immune checkpoint therapeutics.
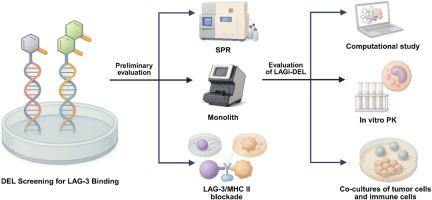
LAGi-DEL的发现和验证:一种在肿瘤免疫共培养模型中逆转免疫抑制的纳米摩尔LAG-3抑制剂
淋巴细胞活化基因-3 (LAG-3)是一种免疫检查点受体,可驱动T细胞衰竭和肿瘤免疫逃避。虽然基于抗体的LAG-3抑制剂已经进入临床实践,但它们的肿瘤穿透性差、制造成本高、半衰期长限制了给药的灵活性,并可能导致延长的免疫相关毒性。有效的小分子LAG-3抑制剂仍然难以捉摸,在免疫肿瘤学领域留下了重大空白。在这里,我们报告了通过筛选42亿个化合物dna编码化学文库(DEL)发现的第一个纳米摩尔小分子LAG-3抑制剂LAGi-DEL的发现。LAGi-DEL直接与LAG-3结合,Kd值为97 nM (SPR)和271 nM (MST), EC50值为138 nM,是目前报道的抗LAG-3的最高效价。分子对接和动力学模拟表明,LAGi-DEL诱导了LAG-3结合口袋内稳定的构象变化。在功能上,LAGi-DEL在急性髓系白血病和肺癌共培养模型中恢复T细胞活化,提高IFN-γ分泌,并促进免疫介导的杀伤,其疗效与fda批准的抗体relatlimab相当。药代动力学分析显示了良好的药物样特性,包括高溶解度,可接受的渗透性,强大的代谢和血浆稳定性,对正常细胞的低细胞毒性,以及可忽略的hERG和CYP负担。这些特性,结合广泛的功能活性和前所未有的效力,使LAGi-DEL成为开发口服可用的LAG-3抑制剂的有希望的支架,并作为下一代免疫检查点治疗的蓝图。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


