Synthesis, SAR and Biophysical Mechanism of Action of Cyclopropabenzindole-Pyridinobenzodiazepine (CBI-PDD) Guanine-Adenine DNA Cross-Linking Agents
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
This study investigates the Structure–Activity Relationship (SAR) and biophysical mechanism of action of a series of novel Cyclopropabenzindole-Pyridinobenzodiazepine (CBI-PDD) agents designed to alkylate adenine and guanine bases in the DNA minor groove. A library of 18 analogs was synthesized using a modular approach to vary linker length, rigidity and the CBI and PDD core structures. Structural modifications included alkyl, aromatic, heteroaromatic and sterically constrained linkers, as well as prodrug variants. Several compounds demonstrated potent in vitro cytotoxicity, with lead analogs achieving IC50 values in the low picomolar range across multiple human tumor cell lines. Control compounds with inactivated electrophilic groups in either or both pharmacophores showed markedly reduced activity, confirming the necessity of dual alkylation for optimal cytotoxic potency. Fluorescence-based thermal denaturation (ΔTm up to 34 °C) and gel-based assays were used to assess DNA binding and provide mechanistic insights into the DNA sequence requirements for mono-alkylation and interstrand cross-linking. The study also includes a scalable synthesis of a linker-payload construct suitable for Antibody–Drug Conjugate (ADC) applications based on these agents. Overall, these findings support the use of CBI-PDDs as a promising class of sequence-selective DNA cross-linking agents for targeted cancer therapies.
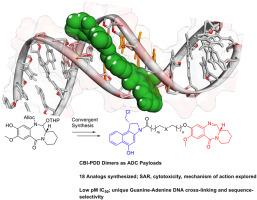
环丙苯并吲哚-吡啶苯并二氮卓(CBI-PDD)鸟嘌呤-腺嘌呤DNA交联剂的合成、SAR及生物物理作用机理
本研究研究了一系列新型环丙苯并吲哚-吡啶苯并二氮卓类药物(CBI-PDD)的构效关系(SAR)和生物物理作用机制。采用模块化方法合成了18个类似物库,以改变连接器的长度、刚度以及CBI和PDD核心结构。结构修饰包括烷基、芳香族、杂芳香族和空间约束连接,以及前药变体。几种化合物显示出强大的体外细胞毒性,铅类似物在多种人类肿瘤细胞系的低皮摩尔范围内达到IC50值。在一个或两个药效团中具有失活的亲电基团的对照化合物显示出明显降低的活性,证实了双烷基化对最佳细胞毒效力的必要性。基于荧光的热变性(ΔTm高达34°C)和基于凝胶的测定用于评估DNA结合,并提供单烷基化和链间交联所需的DNA序列的机制见解。该研究还包括基于这些药物的适用于抗体-药物偶联(ADC)应用的连接-有效载荷结构的可扩展合成。总的来说,这些发现支持将cbi - pdd作为一类有前途的序列选择性DNA交联剂用于靶向癌症治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


