In silico discovery and mechanistic profiling of STING agonists engaging the transmembrane domain
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Stimulator of interferon genes (STING) is an ER resident cytosolic pattern recognition receptor involved in innate immune signaling and is a promising therapeutic target in immuno-oncology and vaccine adjuvant design. While canonical STING agonists typically activate the receptor via direct engagement with the cytosolic cyclic dinucleotide (CDN)-binding domain (CBD), recent high-resolution structural studies have uncovered a distinct allosteric binding site within the transmembrane domain (TMD). Here, we report the identification and characterization of a novel STING agonist, compound 7k, which uniquely engages the TMD rather than the cytosolic domain. Through comparative molecular docking and binding site validation, the TMD of STING was computationally identified as the preferential site of engagement, diverging from the classical CBD. This mode of activation is functionally significant, as it leads to a demonstrably distinct set of downstream molecular phenotypes. Furthermore, our study led to the discovery of structurally related series of potent, small-molecule human STING activators with potential utility as immunomodulatory therapeutics. A lead compound, 7k, emerged with potent STING-dependent activity in vitro and displayed adjuvant efficacy in vivo, as shown by enhanced antigen-specific IgG production and Th1/Th2 cytokine responses in a genetically humanized STING mouse model. These findings support the TMD as a druggable allosteric site and highlight 7k as a promising candidate for next-generation STING-targeted immunotherapeutics.

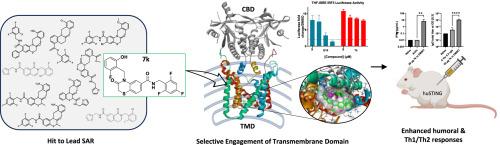
参与跨膜结构域的STING激动剂的硅发现和机制分析
干扰素基因刺激因子(STING)是一种内质网驻留的细胞质模式识别受体,参与先天免疫信号传导,是免疫肿瘤学和疫苗佐剂设计中有前景的治疗靶点。虽然典型的STING激动剂通常通过直接与细胞质环二核苷酸(CDN)结合域(CBD)结合来激活受体,但最近的高分辨率结构研究发现了跨膜结构域(TMD)内独特的变构结合位点。在这里,我们报告了一种新的STING激动剂,化合物7k的鉴定和表征,它独特地作用于TMD而不是细胞质结构域。通过比较分子对接和结合位点验证,计算确定了STING的TMD为优先结合位点,有别于经典CBD。这种激活模式在功能上是显著的,因为它导致一组明显不同的下游分子表型。此外,我们的研究还发现了一系列结构相关的强效小分子人类STING激活剂,具有潜在的免疫调节治疗用途。在基因人源化的STING小鼠模型中,一种先导化合物7k在体外具有强效的STING依赖活性,并在体内显示出佐剂效果,这可以通过增强抗原特异性IgG的产生和Th1/Th2细胞因子的反应来证明。这些发现支持TMD是一个可药物化的变构位点,并强调7k是下一代sting靶向免疫治疗的有希望的候选者。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


